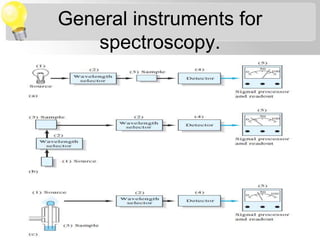
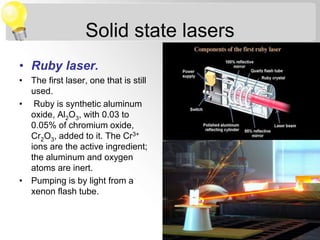
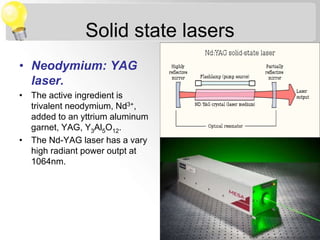
Sana Shaikh is studying for her M.Sc in physical chemistry with a specialization in analytical chemistry. Her topic is on sources of electromagnetic radiation used in spectroscopy. There are two main types of sources - continuum sources that emit over a wide range of wavelengths like tungsten filament lamps, and line sources that emit discrete wavelengths like gas discharge lamps. Lasers are also described as precise line sources that are monochromatic and coherent.