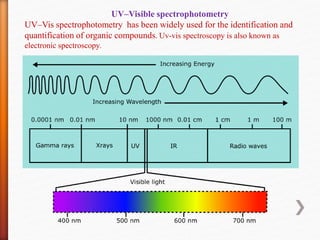
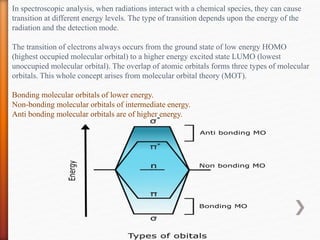
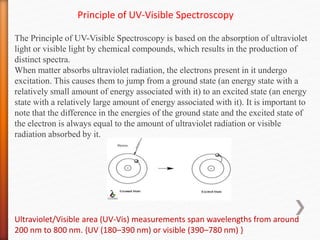
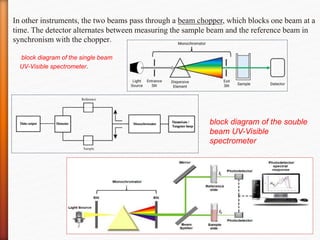
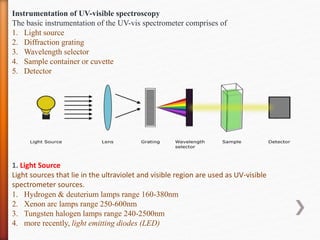
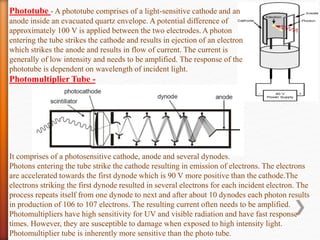
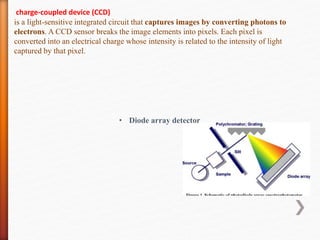
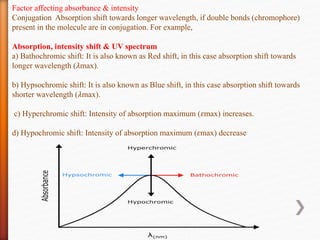
The document discusses ultraviolet-visible (UV-Vis) spectrophotometry, a branch of science focused on the interaction of light with matter, highlighting its use in identifying and quantifying organic compounds. It explains key principles such as the Beer-Lambert law, which relates absorbance to concentration and path length, and describes the instrumentation and components of a UV-Vis spectrophotometer, including light sources, detectors, and sample containers. Additionally, it covers the applications of UV-Vis spectroscopy in purity detection, structural elucidation, and quantitative determinations in chemistry.