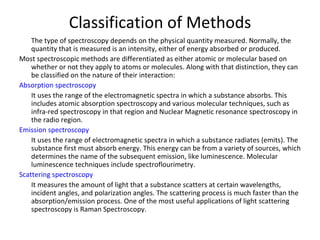
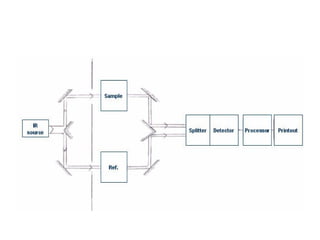
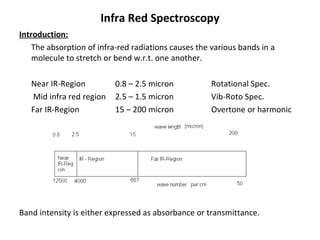
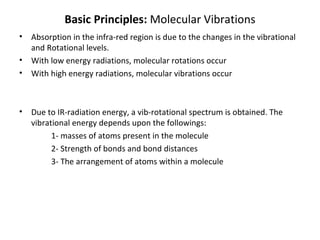
1. Infrared spectroscopy analyzes molecular vibrations and rotations that occur when molecules absorb infrared radiation.
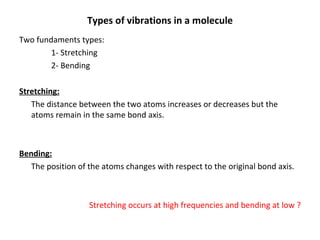
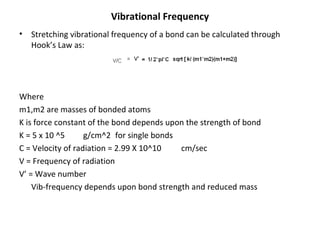
2. Different types of molecular vibrations like stretching and bending occur at characteristic frequencies that can identify functional groups and molecular structure.
3. The document discusses various spectroscopic techniques like fluorescence, X-ray, UV-Vis, IR, Raman, and NMR spectroscopy and their applications in chemistry.