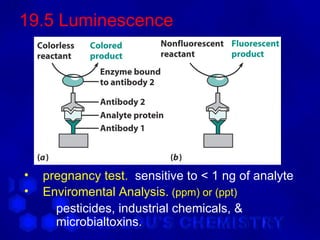
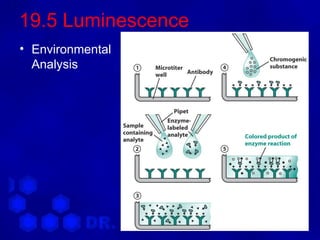
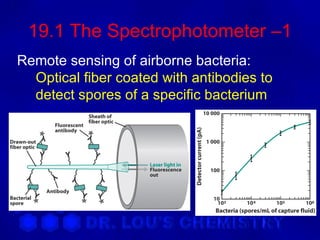
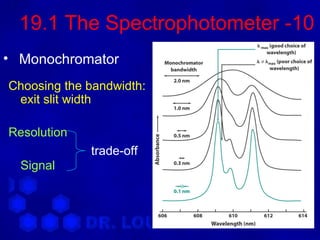
This document discusses spectrophotometry and luminescence techniques. It describes the basic components of a spectrophotometer including the light source, monochromator, and detector. The monochromator uses prisms or diffraction gratings to separate wavelengths and can be used to measure absorption across the spectrum. Luminescence techniques like fluorescence and phosphorescence can provide information about electronic transitions and are used in analytical applications like immunoassays and environmental analysis due to their high sensitivity.















![19.2 Analysis of a mixture -1
• Absorbance of a mixture :
A = exb[X] + eyb[Y] + …](https://image.slidesharecdn.com/chapter19-120603234240-phpapp01/85/Chapter-19-18-320.jpg)

![19.2 Analysis of a mixture -3
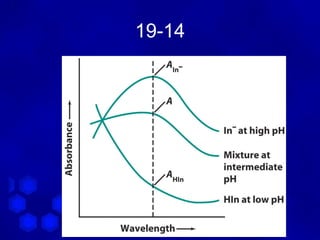
Why isosbestic point?
A 465 = ε 465 [ HIn ]
HIn
[ ]
A 465 = ε 465 In −
In −
when [ HIn ] = [ In ] ⇒ ε
− 465
HIn = ε 465 = ε 465
In −
∴ For a mixture :
HIn In −
[ ]
A 465 = ε 465 b [ HIn ] + ε 465 b In −
= ε 465 b ( [ In ] + [ HIn ] )
− −](https://image.slidesharecdn.com/chapter19-120603234240-phpapp01/85/Chapter-19-20-320.jpg)

![19.3 Spectrophotometric Titrations-2
Ferric nitrilotriacetate
[used to avoid Fe(OH)3 ]](https://image.slidesharecdn.com/chapter19-120603234240-phpapp01/85/Chapter-19-22-320.jpg)