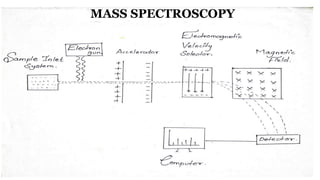
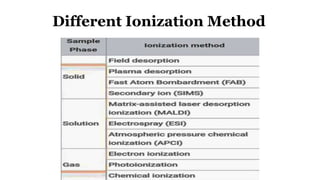
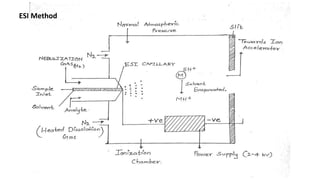
The document discusses electrospray ionization (ESI) and its application in mass spectrometry, an analytical technique used to measure the mass-to-charge ratio of ions generated from samples. ESI enables the analysis of high molecular weight biomolecules by converting samples into ionized droplets, which are then analyzed for their characteristics and structure. It highlights the uses of mass spectrometry in identifying compounds, determining their structure, and applications in various fields like pharmacokinetics and space exploration.