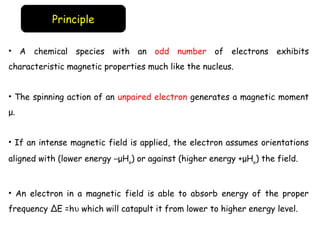
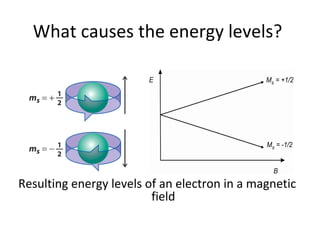
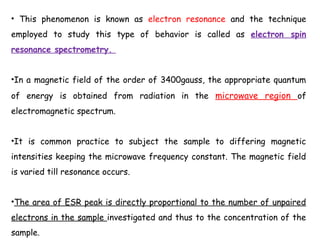
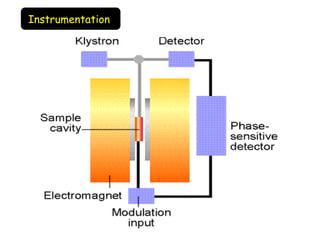
Electron spin resonance (ESR) spectroscopy involves applying a magnetic field to a chemical species with unpaired electrons and measuring the absorption of microwave radiation, which causes transitions between spin energy levels. ESR provides information about electron environments and can be used to study metalloproteins and incorporate spin labels to probe protein structure and dynamics. The instrumentation required includes an electromagnet, microwave source, and detector, along with components to sweep the magnetic field and modulate the signal.





![• ESR spectrometry is one of the main methods to study transition metal
containing metalloproteins.
• Biological macromolecules which lack unpaired electrons cannot be
studied by ESR because they do not resonate.
•Information obtained from ESR spectra:
•1] Rate of catalysis
•2] Active site geometry
•3] Denaturation and protein folding
•4] Enzyme-ligand interaction
APPLICATIONS](https://image.slidesharecdn.com/esrfinal-160521064801/85/ESR-20-320.jpg)