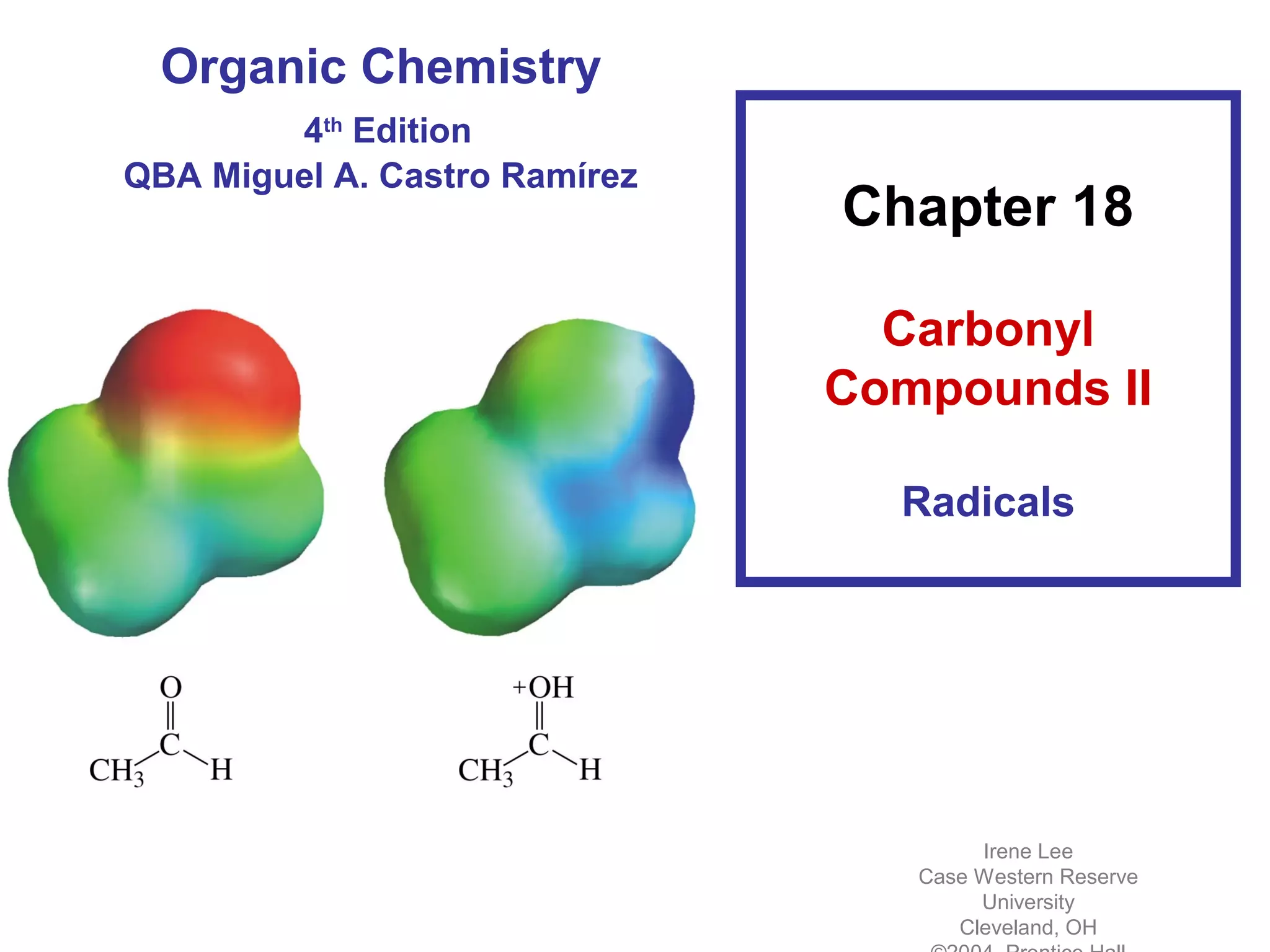
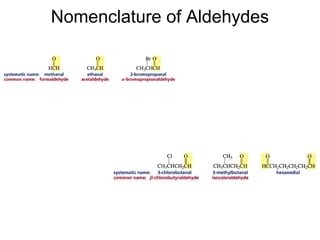
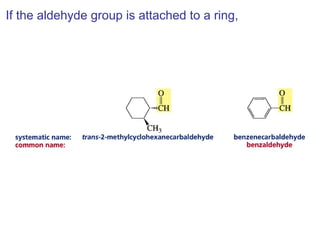
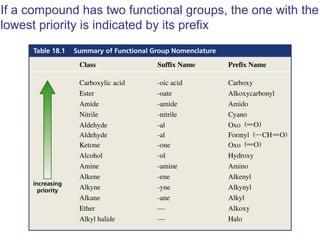
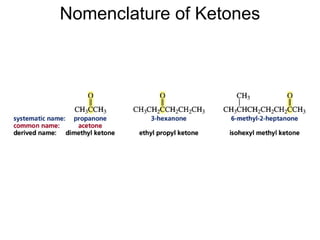
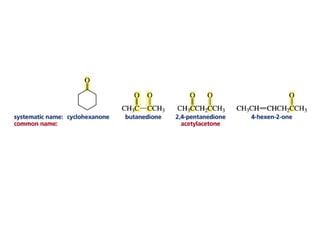
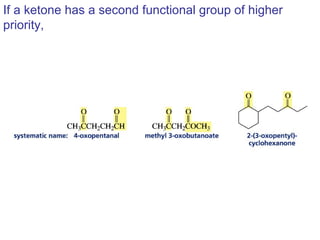
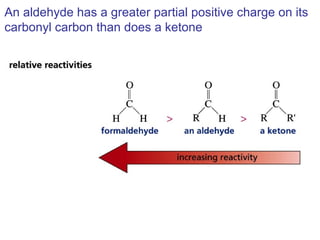
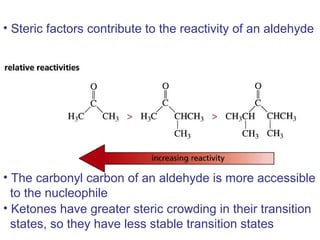
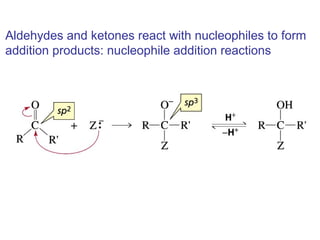
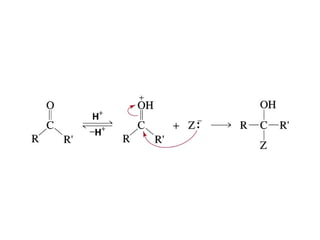
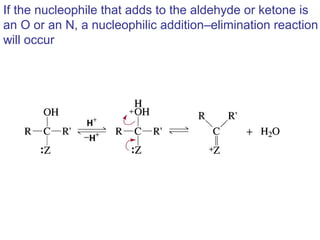
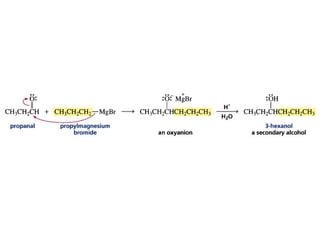
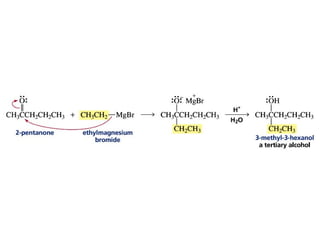
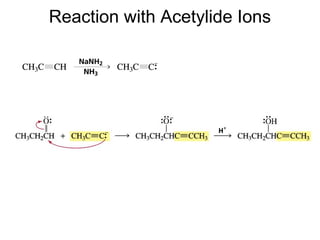
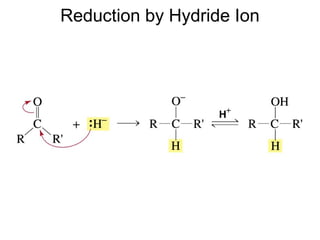
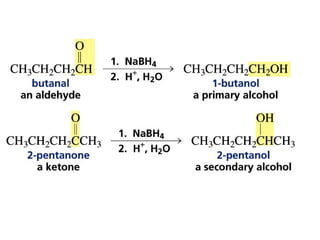
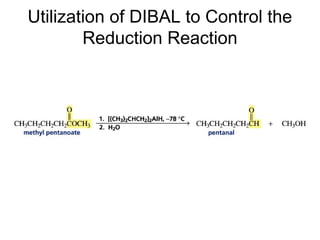
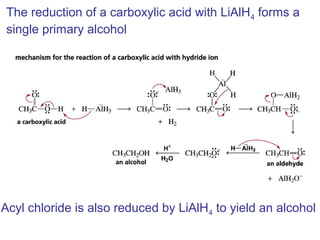
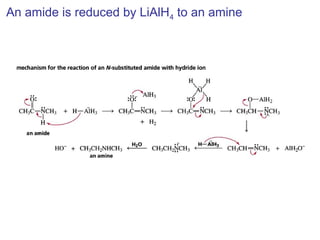
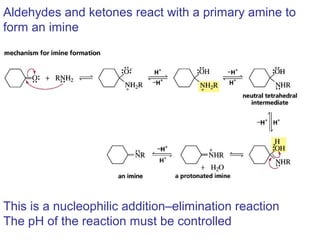
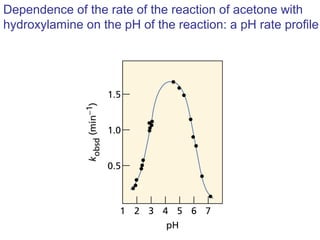
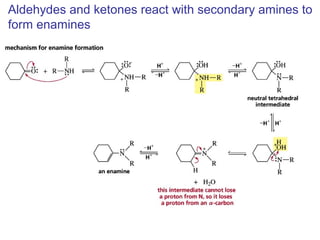
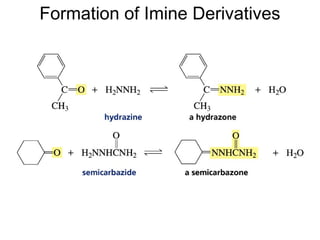
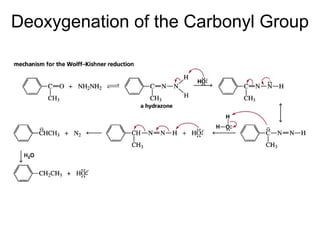
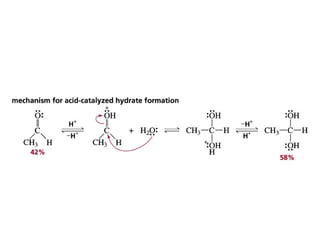
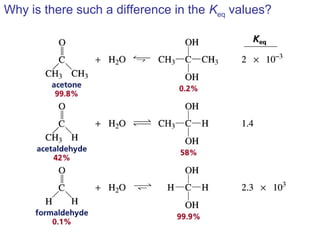
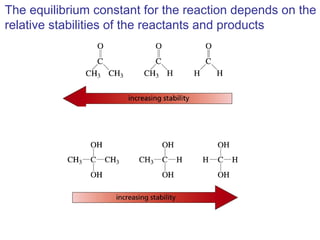
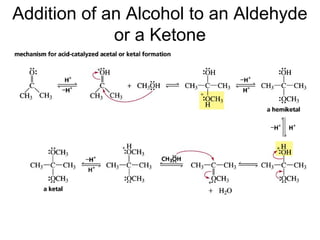
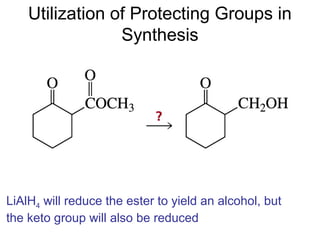
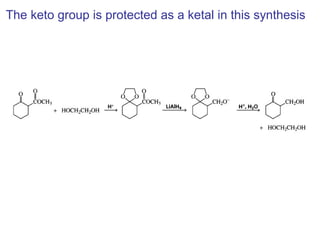
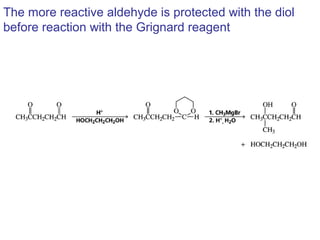
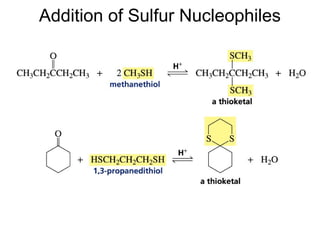
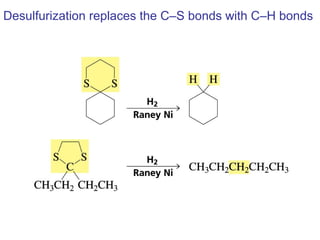
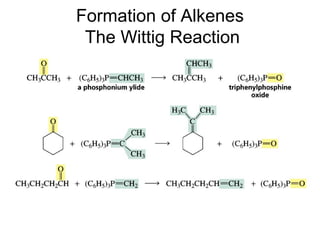
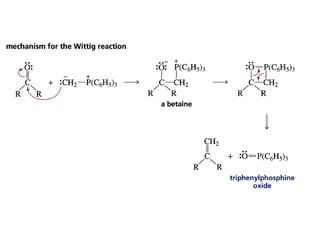
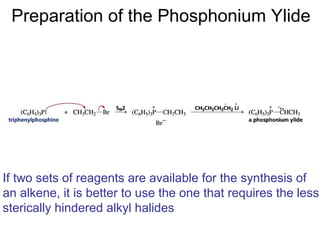
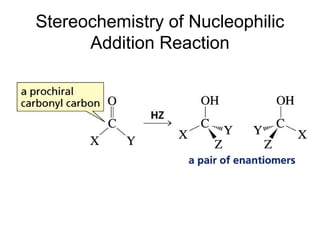
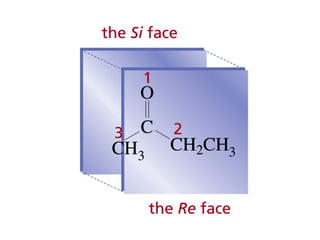
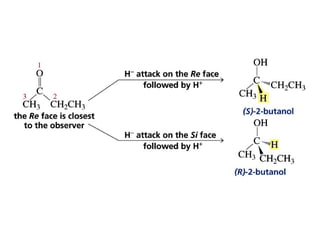
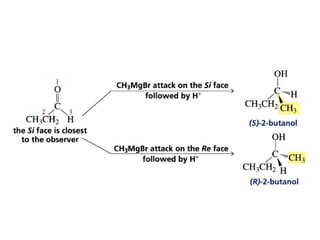
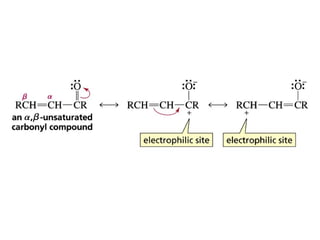
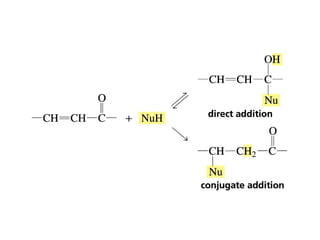
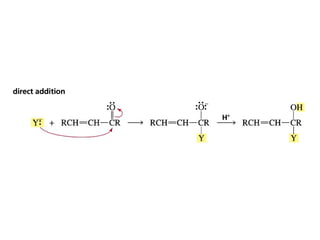
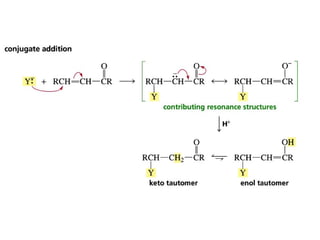
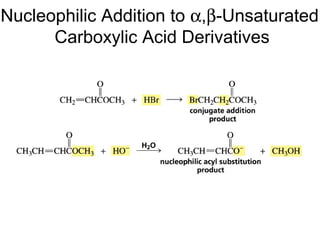
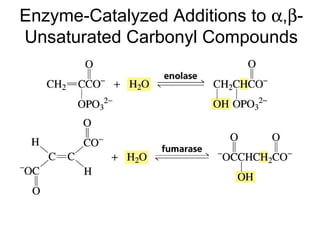
This chapter discusses carbonyl compounds, including aldehydes and ketones. It covers nomenclature, reactivity, and reactions of carbonyl compounds. Specifically, it describes how aldehydes are more reactive than ketones due to increased partial positive charge and steric accessibility. It also summarizes key reactions such as addition, reduction, substitution involving nucleophiles, protecting groups, and stereochemistry of additions.