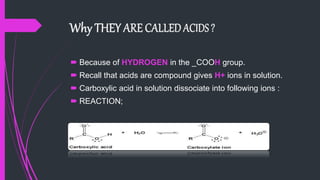
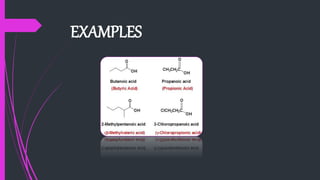
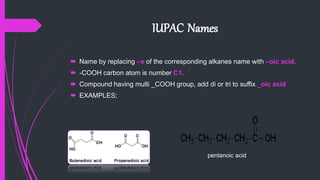
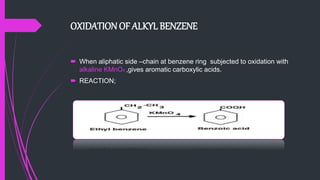
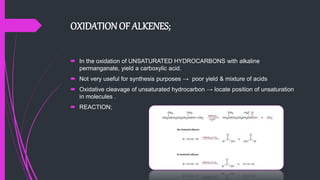
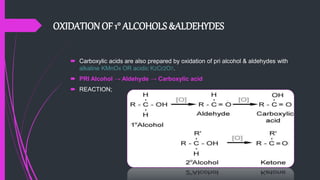
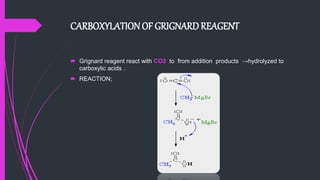
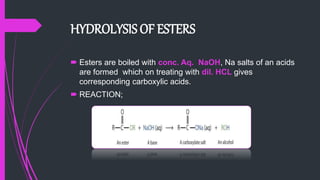
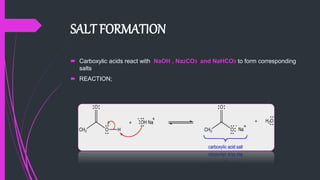
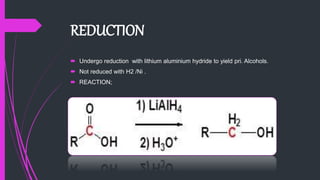
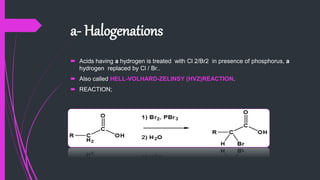
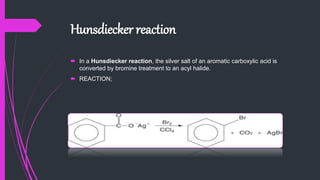
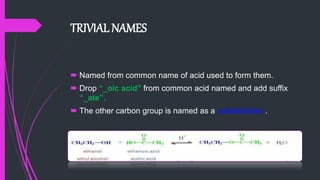
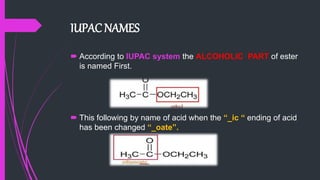
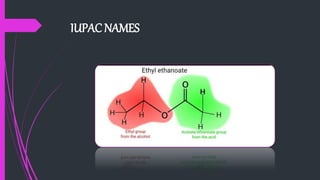
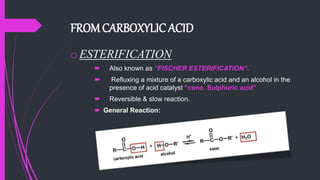
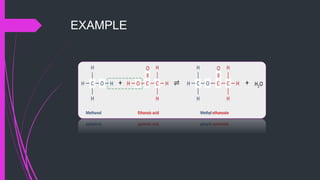
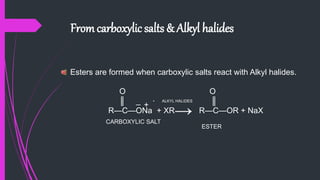
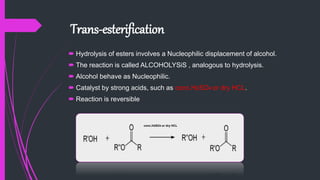
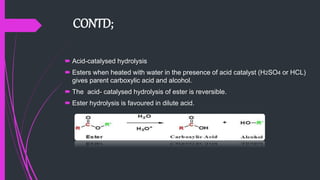
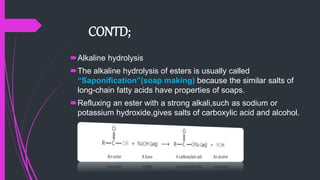
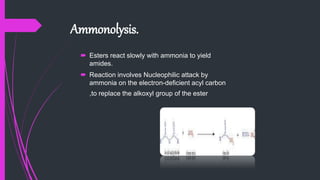
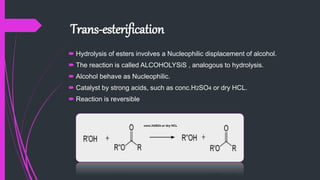
The document provides a comprehensive overview of carboxylic acids, covering their introduction, structure, nomenclature, preparations, physical and chemical properties, and pharmaceutical uses. It details methods for synthesizing carboxylic acids and their derivatives, such as esters, amides, and acyl chlorides, including various chemical reactions involved in these processes. Additionally, it discusses the applications of specific carboxylic acids, like formic and acetic acids, in industrial and pharmaceutical contexts.