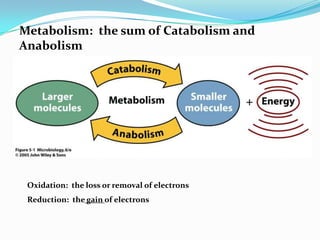
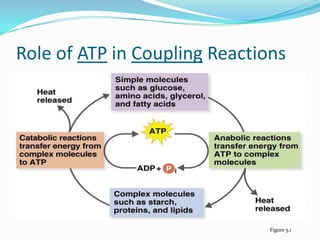
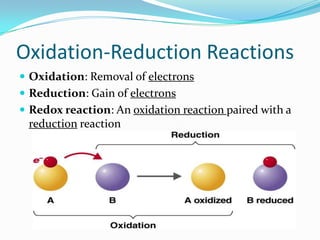
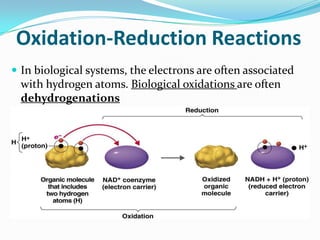
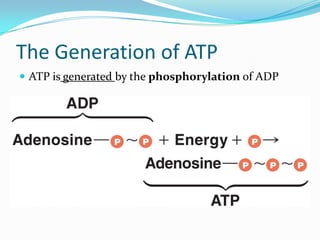
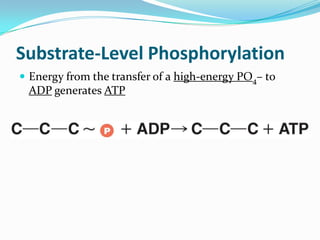
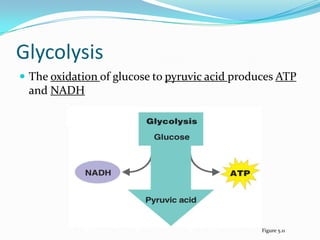
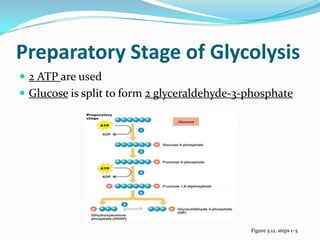
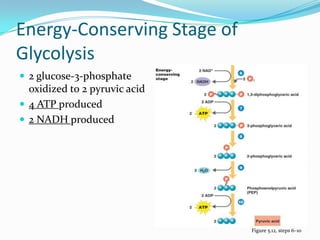
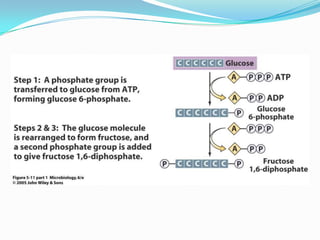
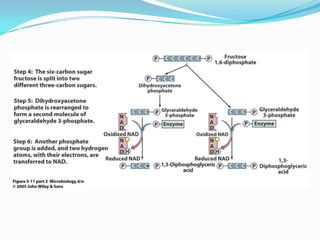
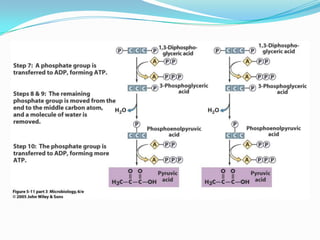
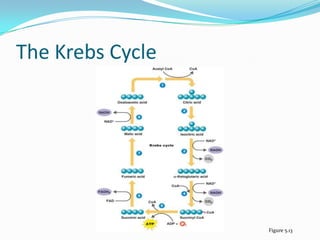
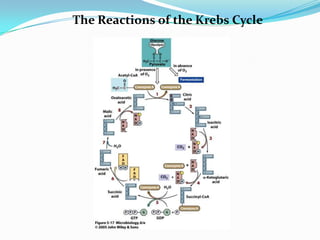
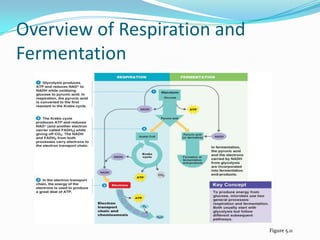
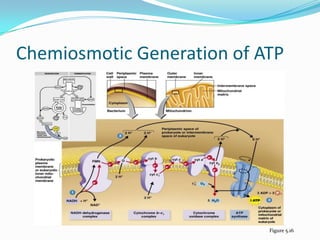
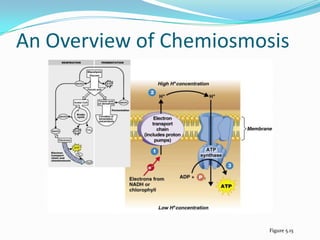
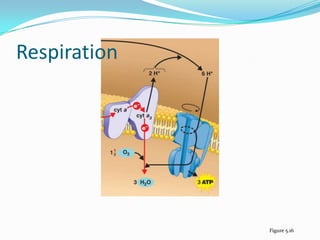
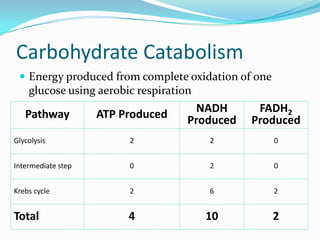
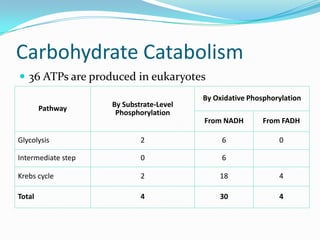
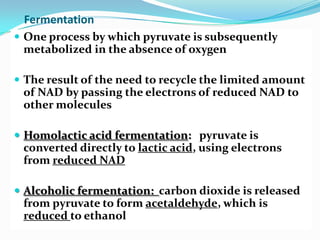
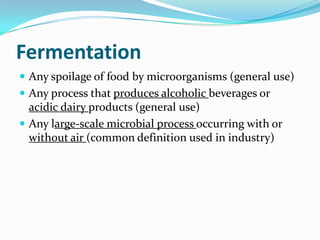
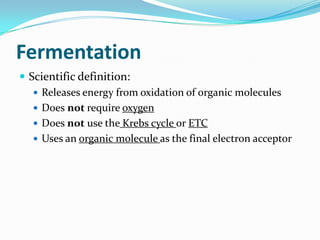
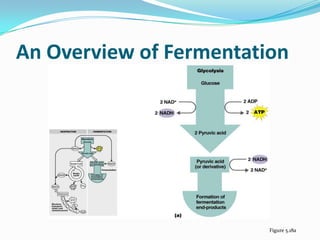
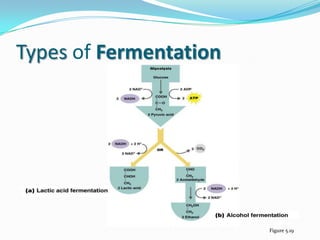
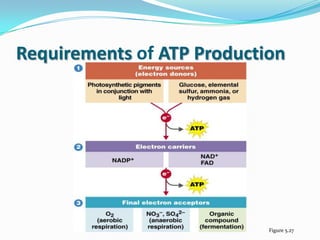
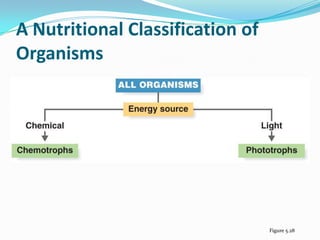
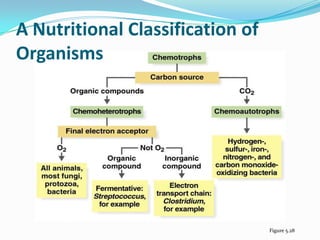
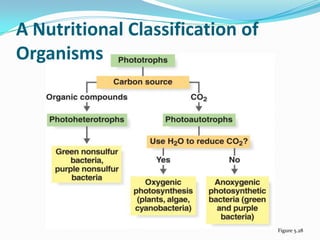
Metabolism involves catabolic and anabolic reactions. Catabolism breaks down molecules to generate energy, while anabolism uses energy to build molecules. Glycolysis and the Krebs cycle oxidize glucose to produce ATP and NADH/FADH2. During oxidative phosphorylation, electrons are passed through an electron transport chain to power ATP synthesis. Fermentation occurs without oxygen and regenerates NAD+ by converting pyruvate into other products like lactic acid or ethanol. Organisms are classified nutritionally based on their energy and carbon sources.