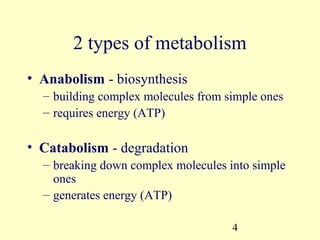
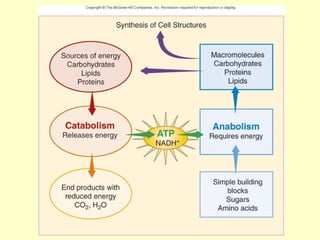
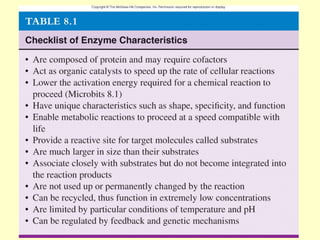
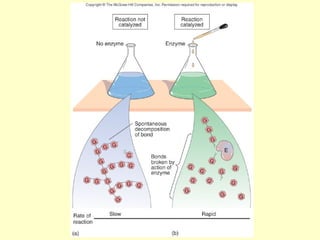
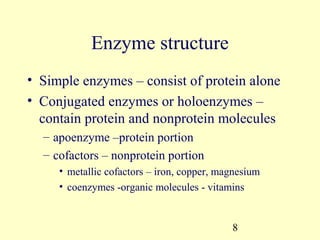
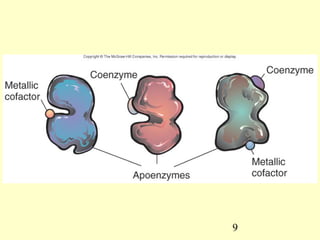
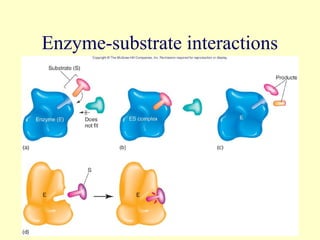
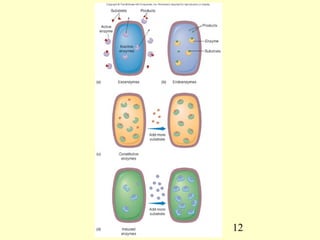
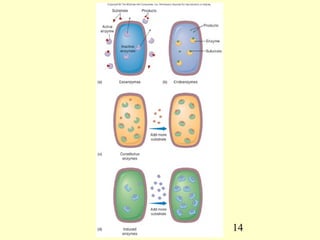
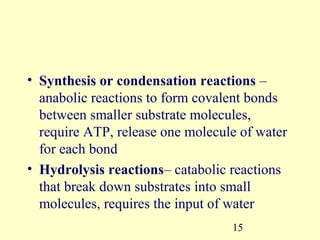
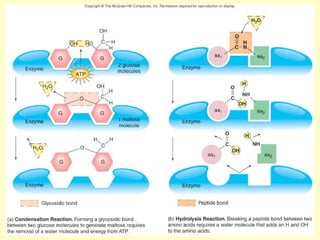
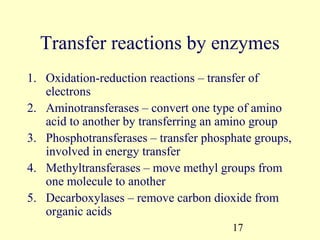
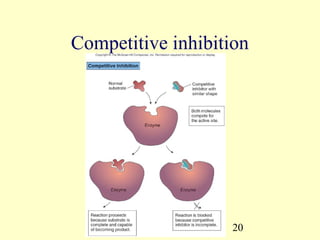
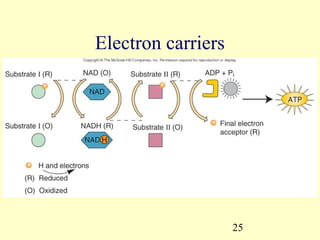
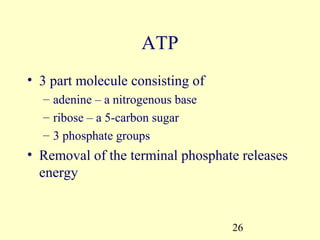
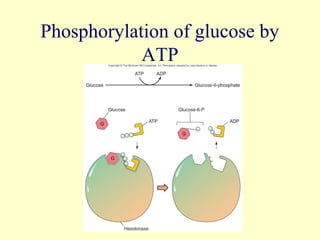
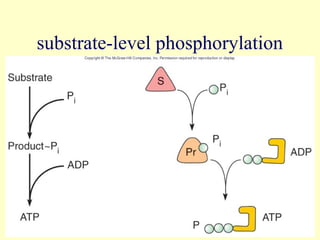
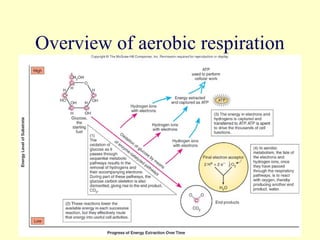
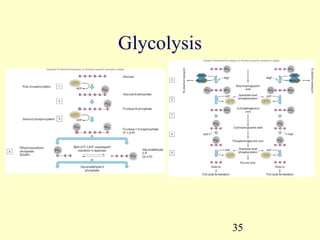
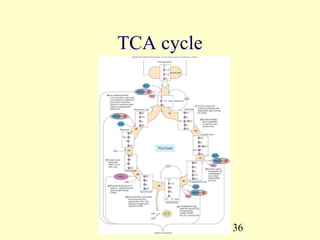
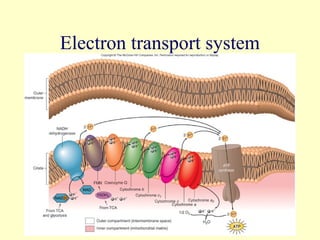
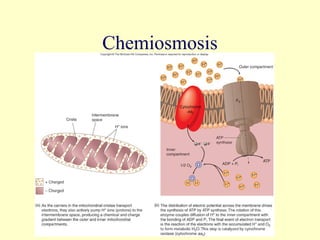
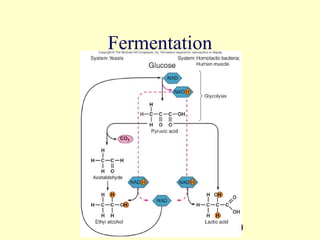
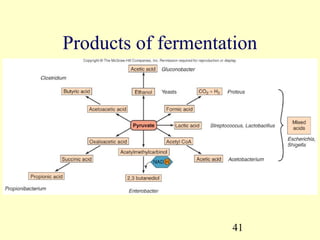
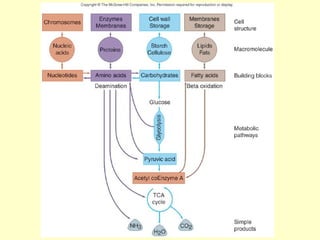
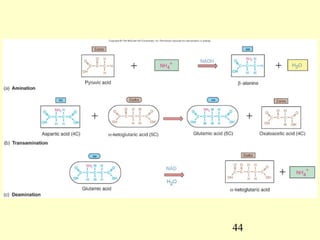
This document provides an overview of microbial metabolism. It discusses the two types of metabolism - anabolism and catabolism. Anabolism involves building complex molecules from simple ones and requires energy, while catabolism breaks down complex molecules into simple ones and generates energy. Key metabolic pathways like glycolysis, the tricarboxylic acid cycle, and the electron transport chain are described. Aerobic respiration and fermentation are compared in terms of the pathways and molecules involved as well as ATP yield. Control mechanisms of enzyme activity like feedback inhibition and induction are also summarized.