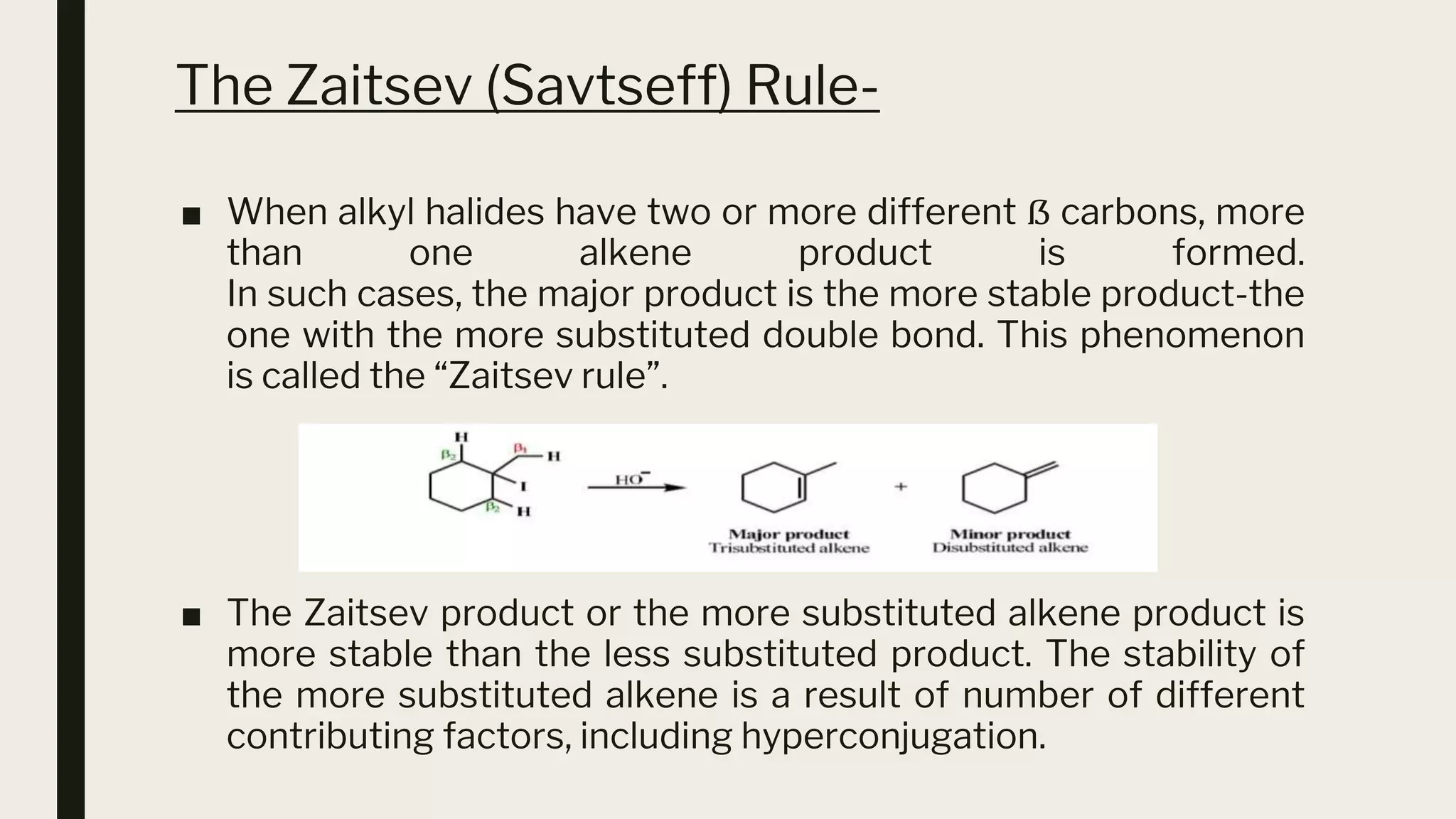
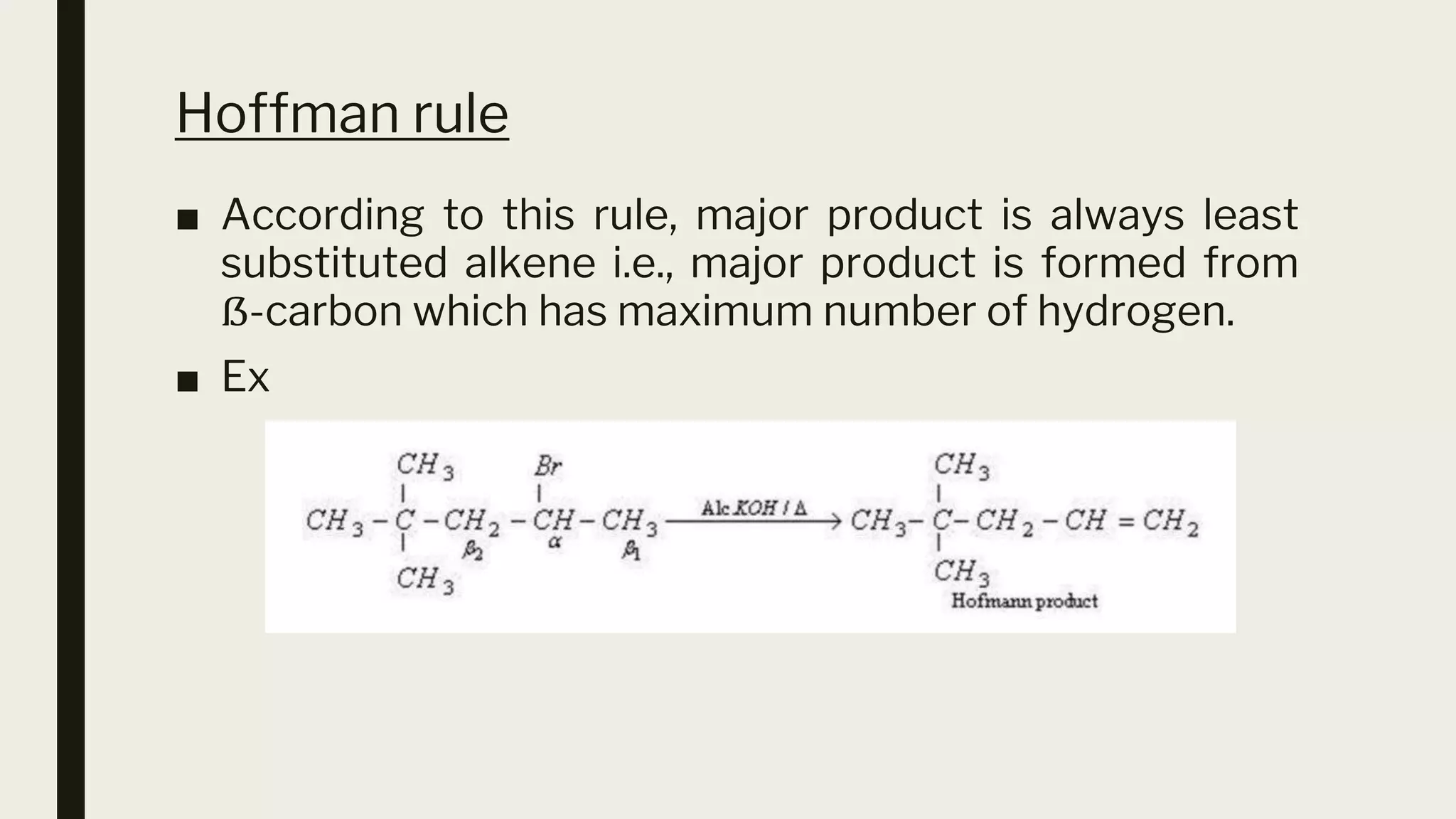
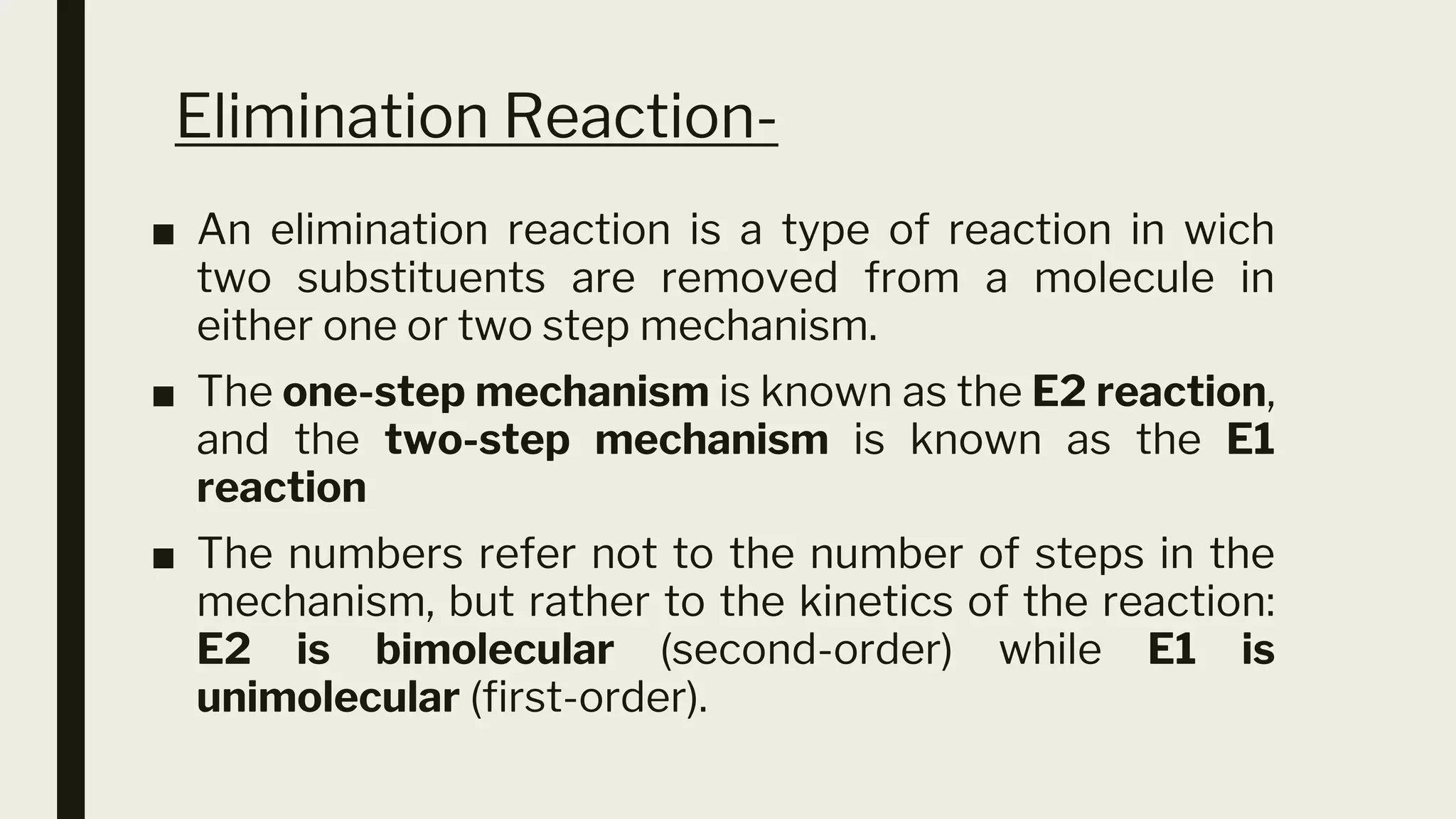
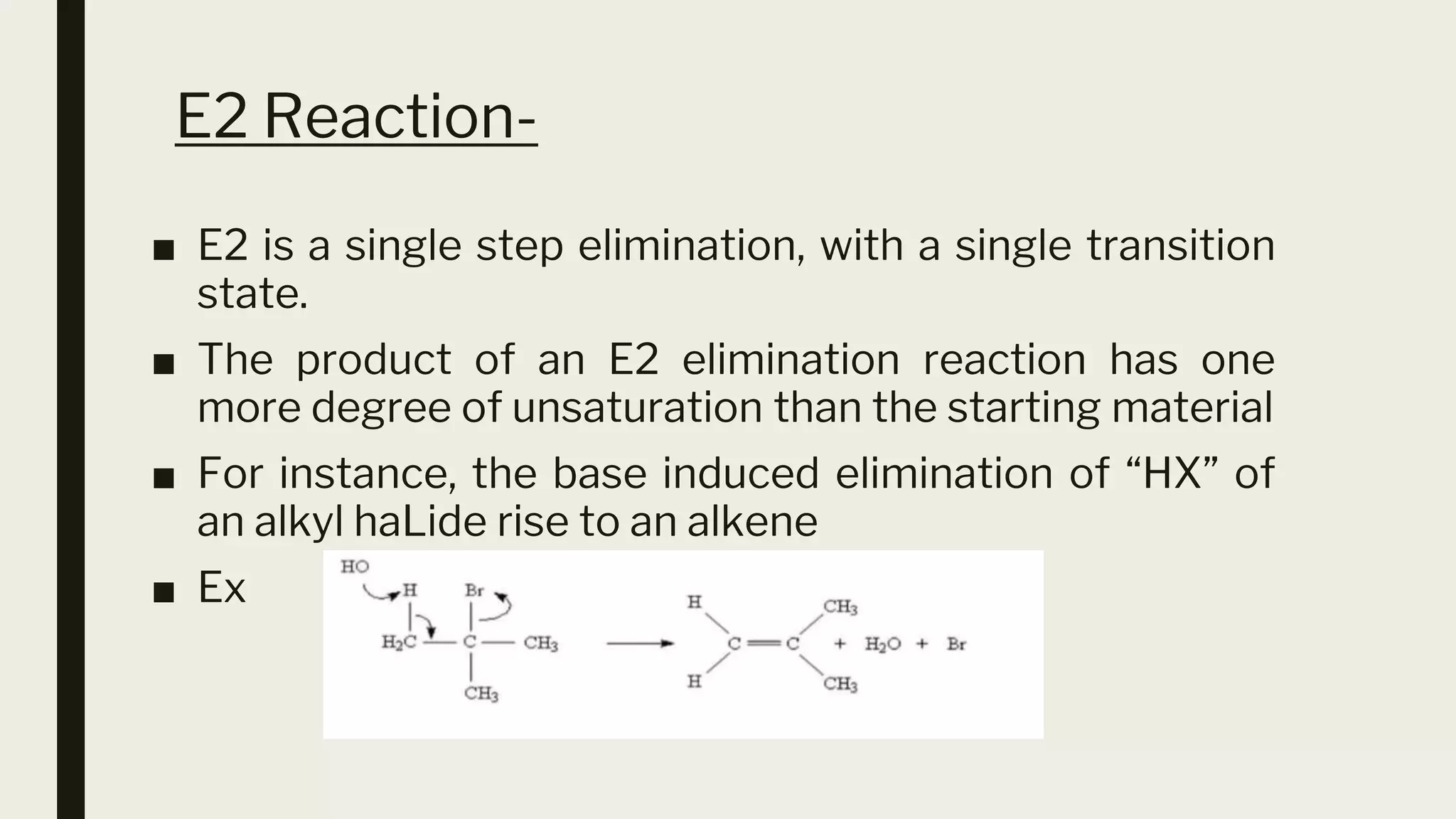
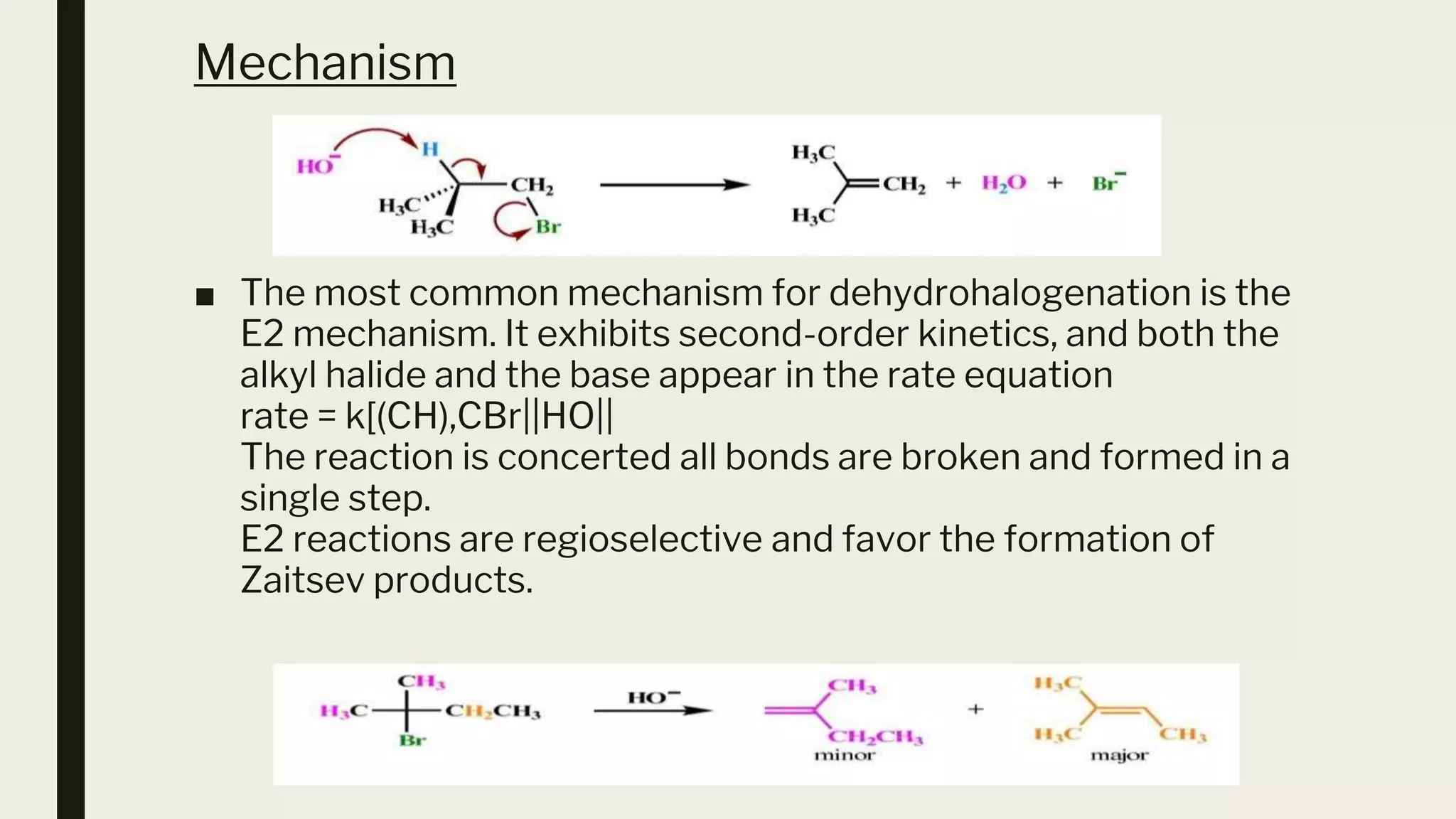
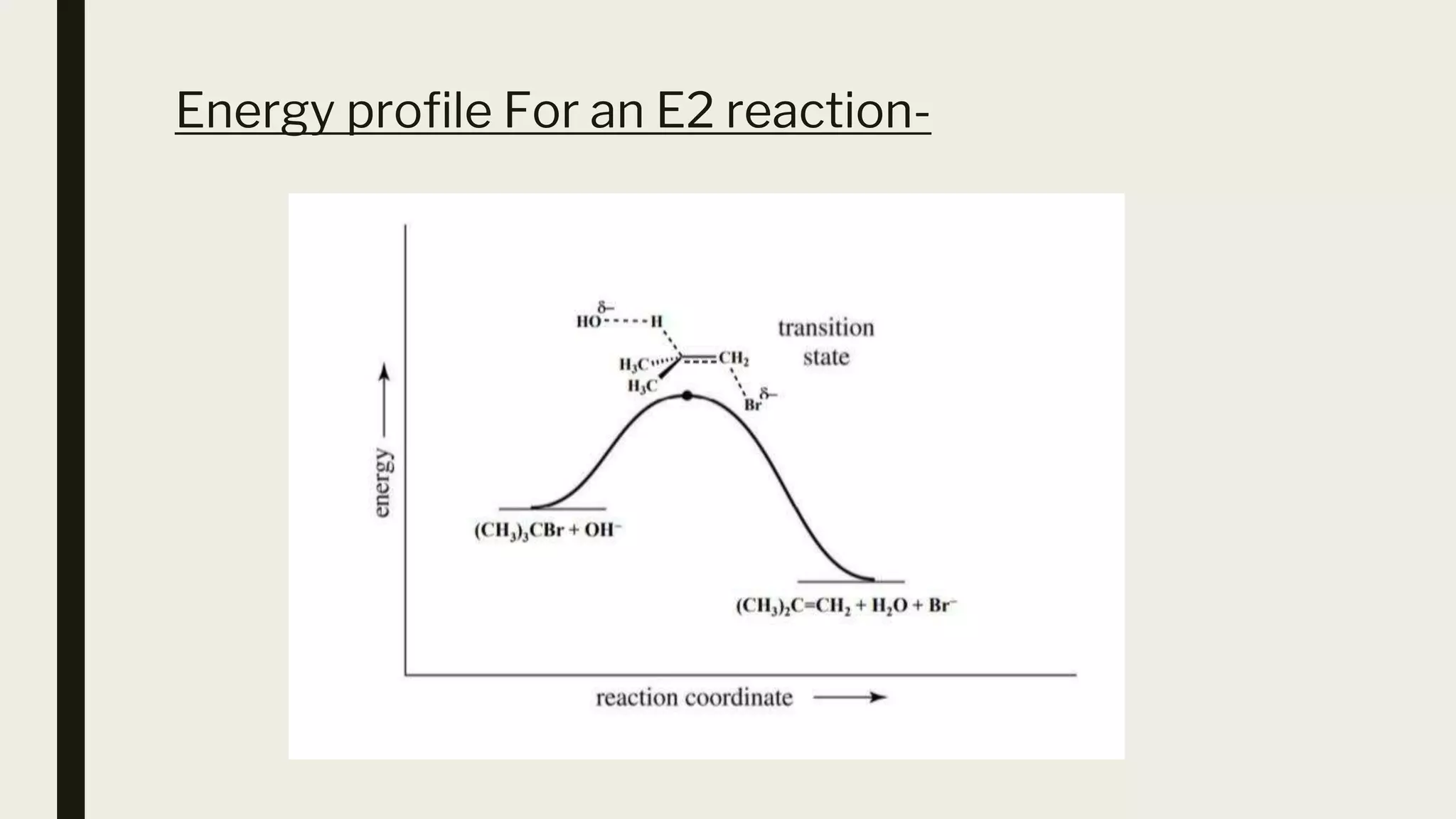
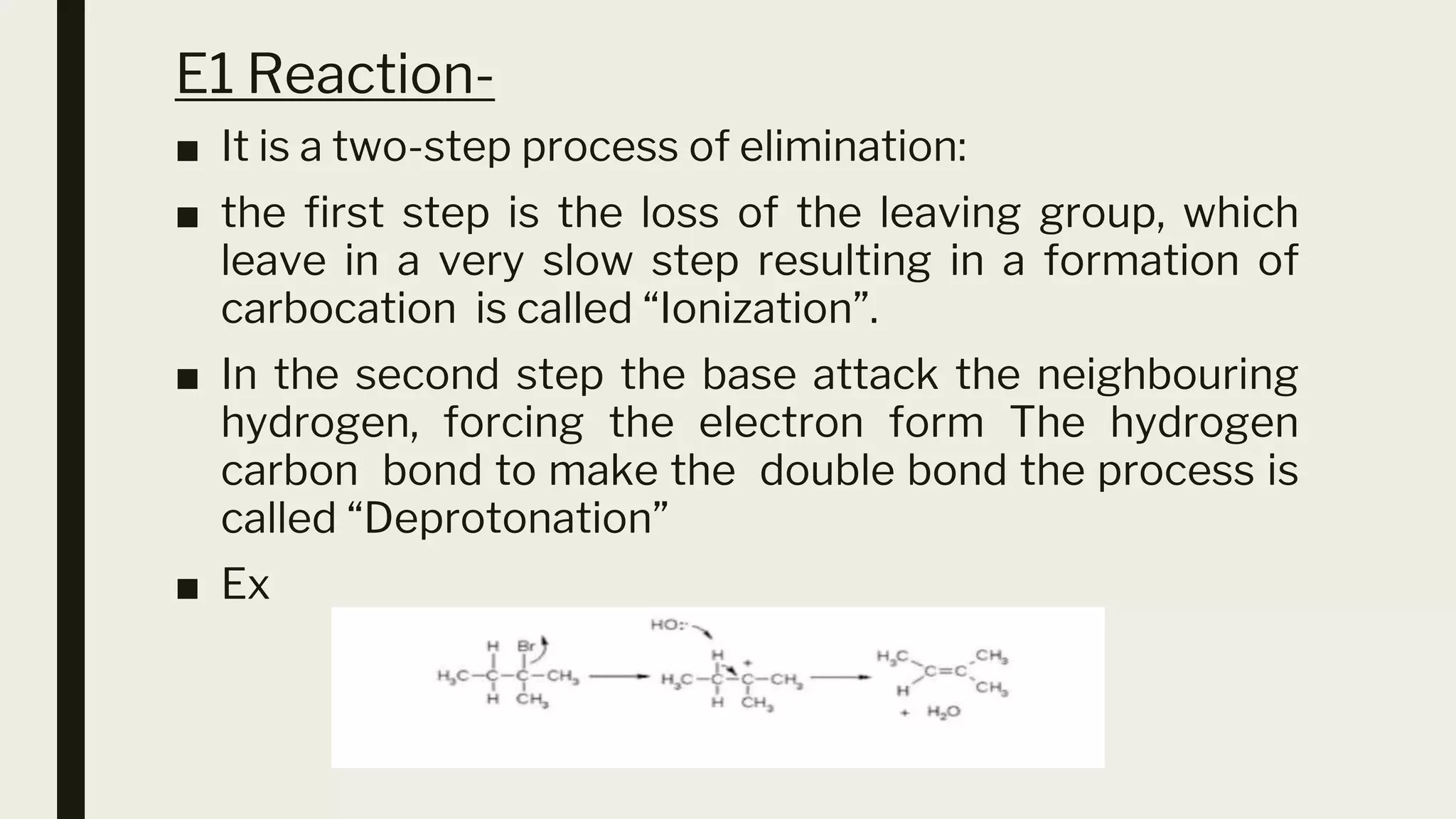
This document discusses elimination reactions, specifically the E1 and E2 mechanisms. The E2 reaction is a single step reaction where a base abstracts two substituents from a molecule to form an alkene. The E1 reaction is a two-step reaction where the leaving group departs first to form a carbocation, followed by deprotonation to form the alkene. Both reactions follow the Zaitsev rule, forming the more substituted alkene product. The Hoffman rule states that the major product comes from the β-carbon with the most hydrogens. Tertiary substrates undergo elimination most readily due to carbocation stability in the E1 and number of β-hydrogens in E2.
![ELIMINATION REACTION [E1 & E2 ; HOFFMAN
& SAYTZEFF’S RULE]
DEPARTMENT OF PHARMACEUTICAL SCIENCES, RTM
NAGPUR UNIVERSITY, NAGPUR
Presented by
Tahmina khan](https://image.slidesharecdn.com/e1e2reactiontkkk-230525025021-534a122b/75/E1-E2-reaction-tkkk-pptx-1-2048.jpg)






![Mechanism-
■ The E1 reaction proceeds via a two-step mechanism: the bond
to the leaving group breaks first before the a bond is formed.
The slow step is unimolecular, involving only the alkyl halide. It
exhibits first-order kinetics,
rate = k[(CH3),CCI]
■ E1 reactions also are regioselective and follow Zaitsev rule](https://image.slidesharecdn.com/e1e2reactiontkkk-230525025021-534a122b/75/E1-E2-reaction-tkkk-pptx-13-2048.jpg)