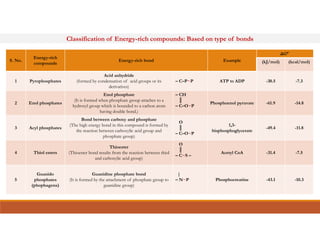
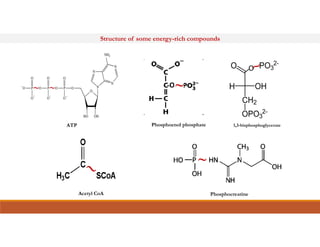
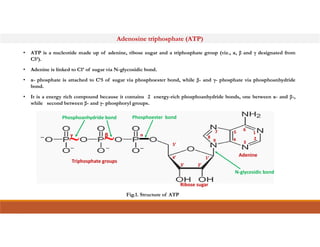
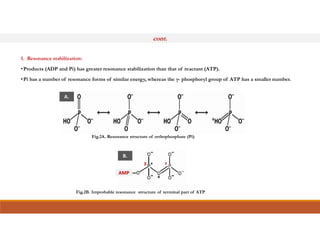
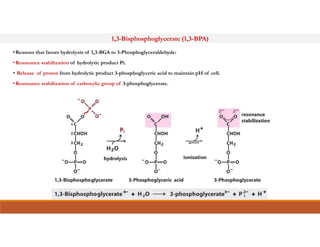
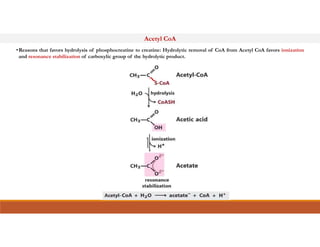
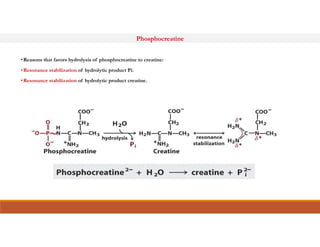
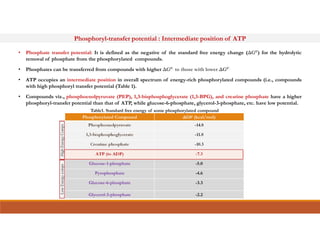
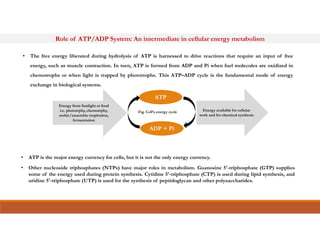
Energy-rich or high-energy compounds in biological systems are those whose hydrolysis yields a free energy (ΔG0') of greater than -25 kJ/mol. These compounds contain high-energy bonds like phosphoanhydride, enol phosphate, acyl phosphate, and guanido phosphate bonds. ATP is an important energy-rich compound that occupies an intermediate position with a ΔG0' of -7.3 kcal/mol for hydrolysis. Its intermediate energy level allows it to accept high-energy phosphoryl groups from compounds like phosphoenolpyruvate and pass them to lower energy compounds. This enables ATP to act as an efficient universal energy carrier in cells.