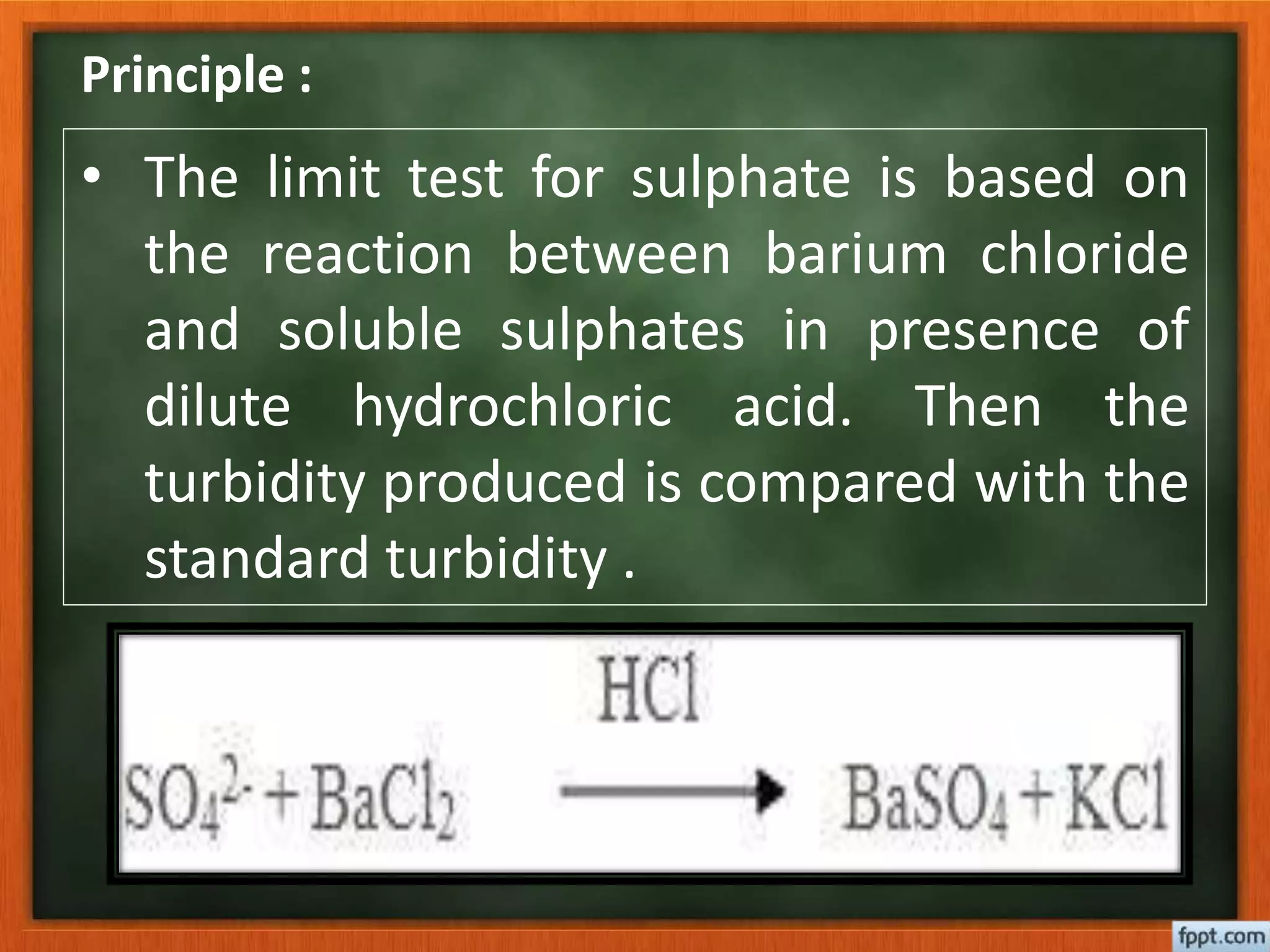
This document describes the procedure for performing a limit test for sulphate according to the Indian Pharmacopoeia. A barium sulphate reagent is prepared containing barium chloride, potassium sulphate, alcohol and water. Standard sulphate solutions are also prepared. The test involves adding nitric acid and the reagent to samples and standards, observing any turbidity formed, and comparing the sample to the standard. If the sample turbidity is less than the standard, it passes the limit test, and if greater, it fails the test.
















![Sample I [ Pass Sample ] :
• Observation:
• The turbidity produced in the test solution is
lesser than standard solution.
• Inference:
• The given substance passes the limit test for
sulphate as per Indian pharmacopoeia when
compared with that of a standard substance.](https://image.slidesharecdn.com/3-170808044620/75/3-limit-test-for-sulphate-18-2048.jpg)
![Sample II [ Fail Sample ] :
• Observation:
• The turbidity produced in the test solution is
more than standard solution.
• Inference:
• The given substance fails the limit test for
sulphate as per Indian pharmacopoeia when
compared with that of a standard substance.](https://image.slidesharecdn.com/3-170808044620/75/3-limit-test-for-sulphate-19-2048.jpg)