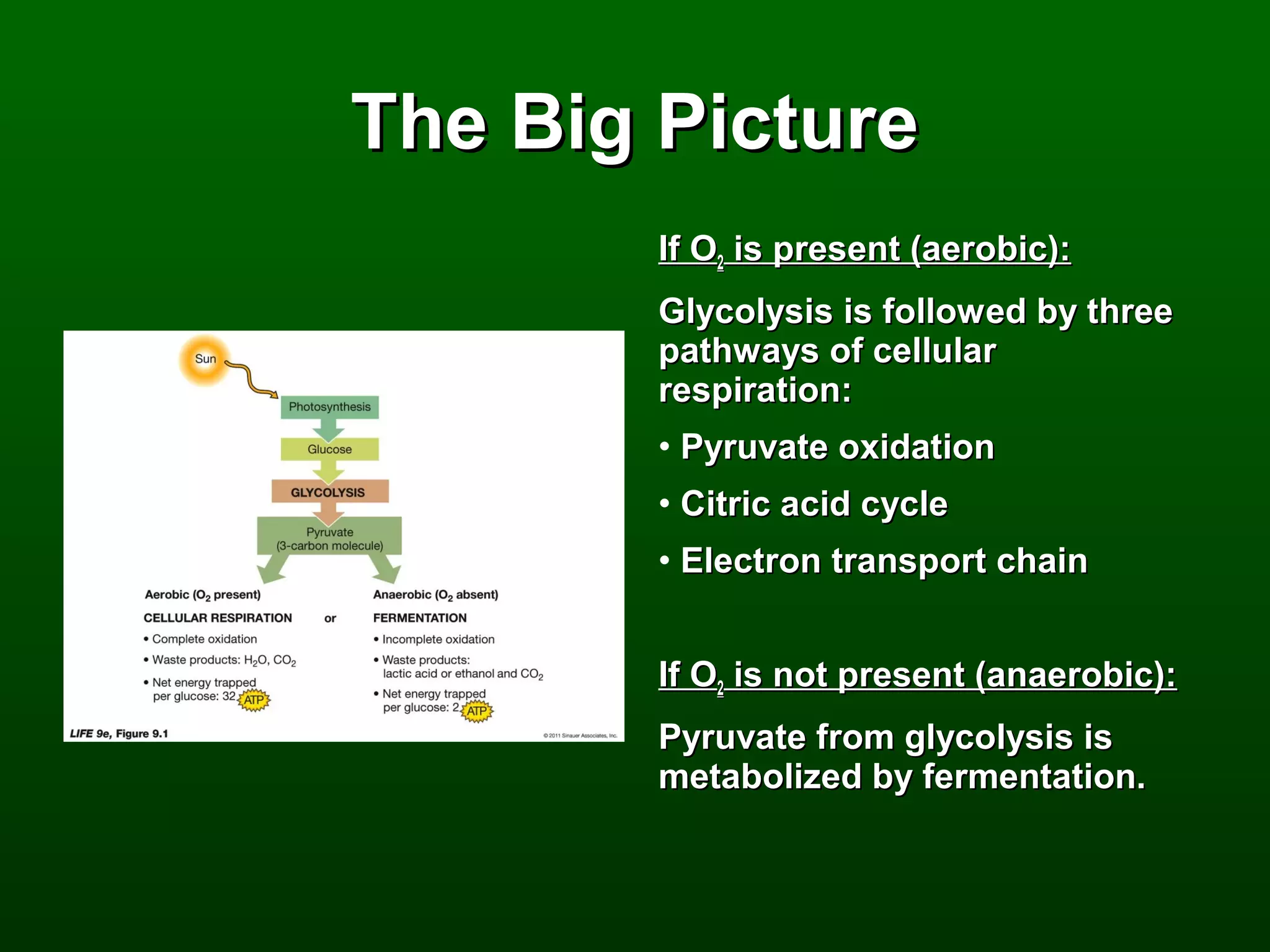
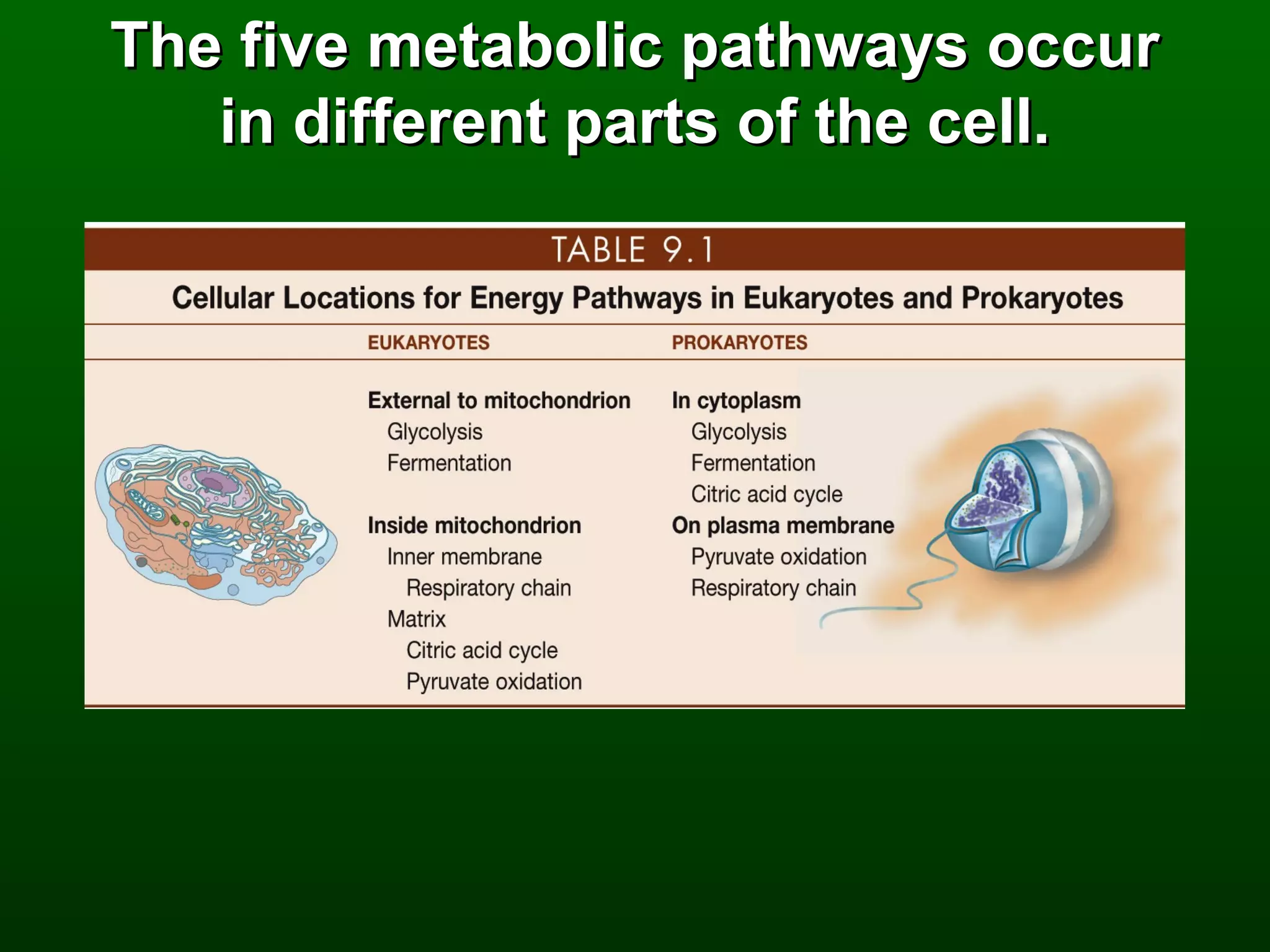
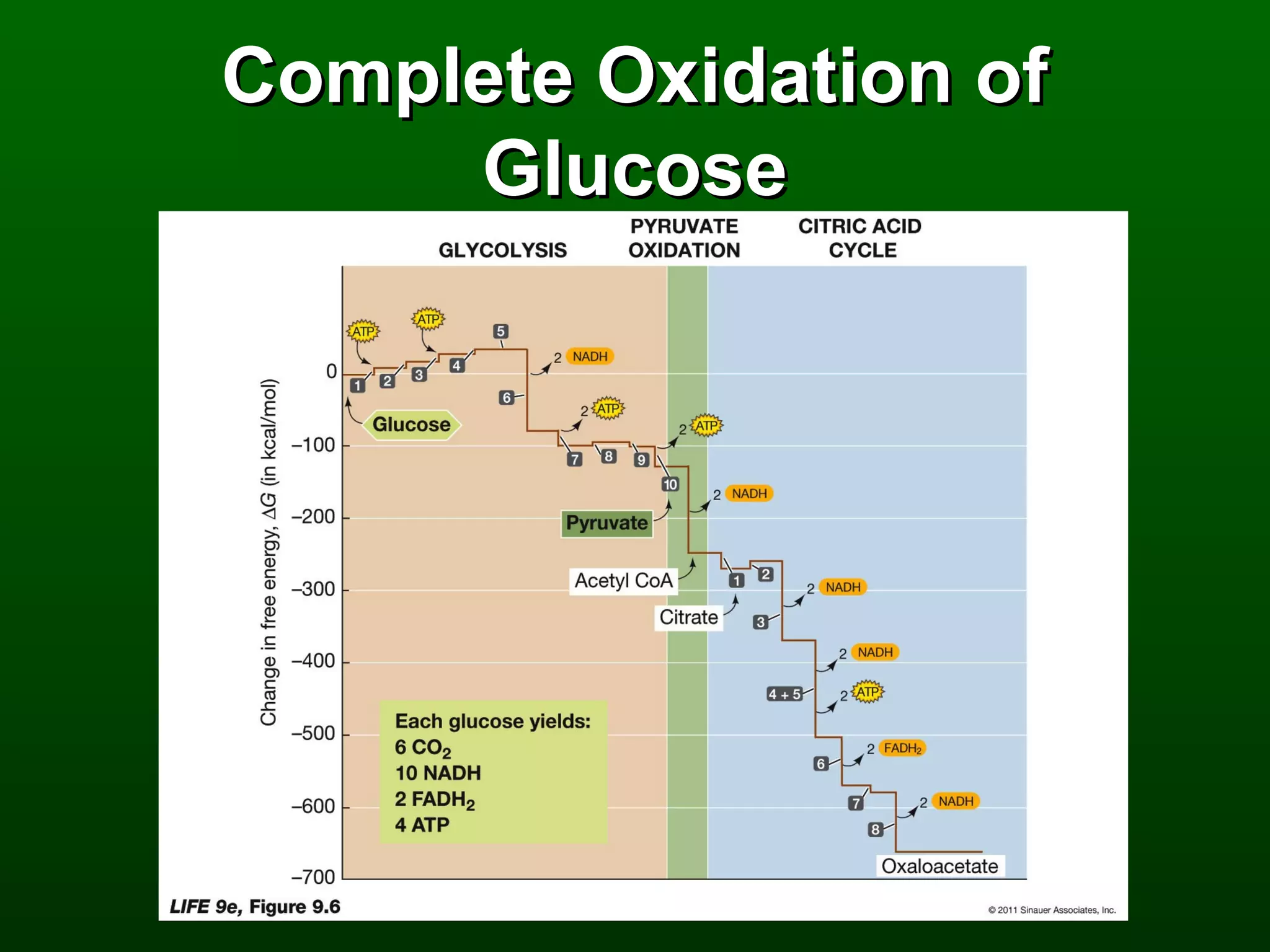
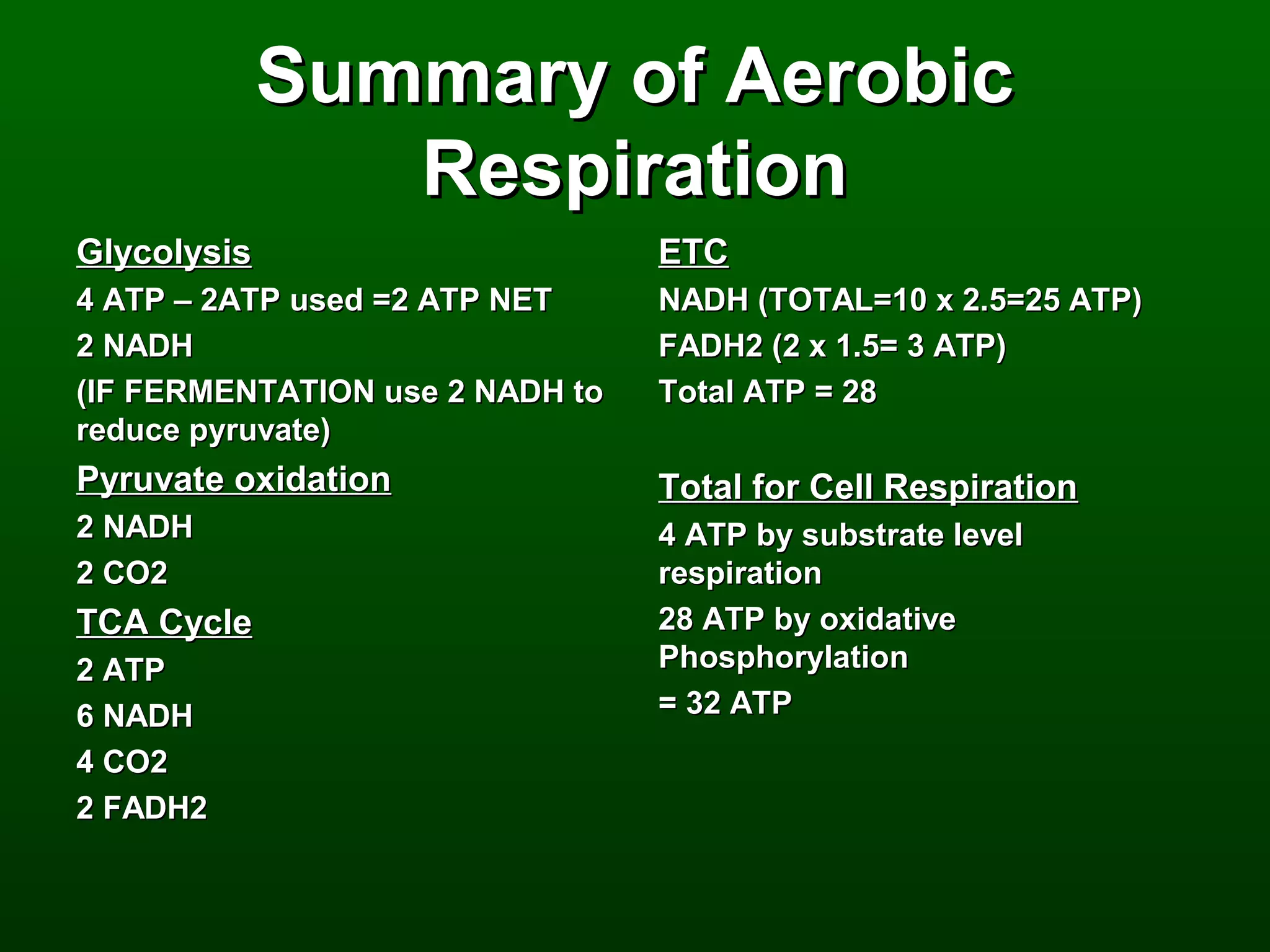
1) The document describes the metabolic pathways that break down glucose to harvest its stored chemical energy.
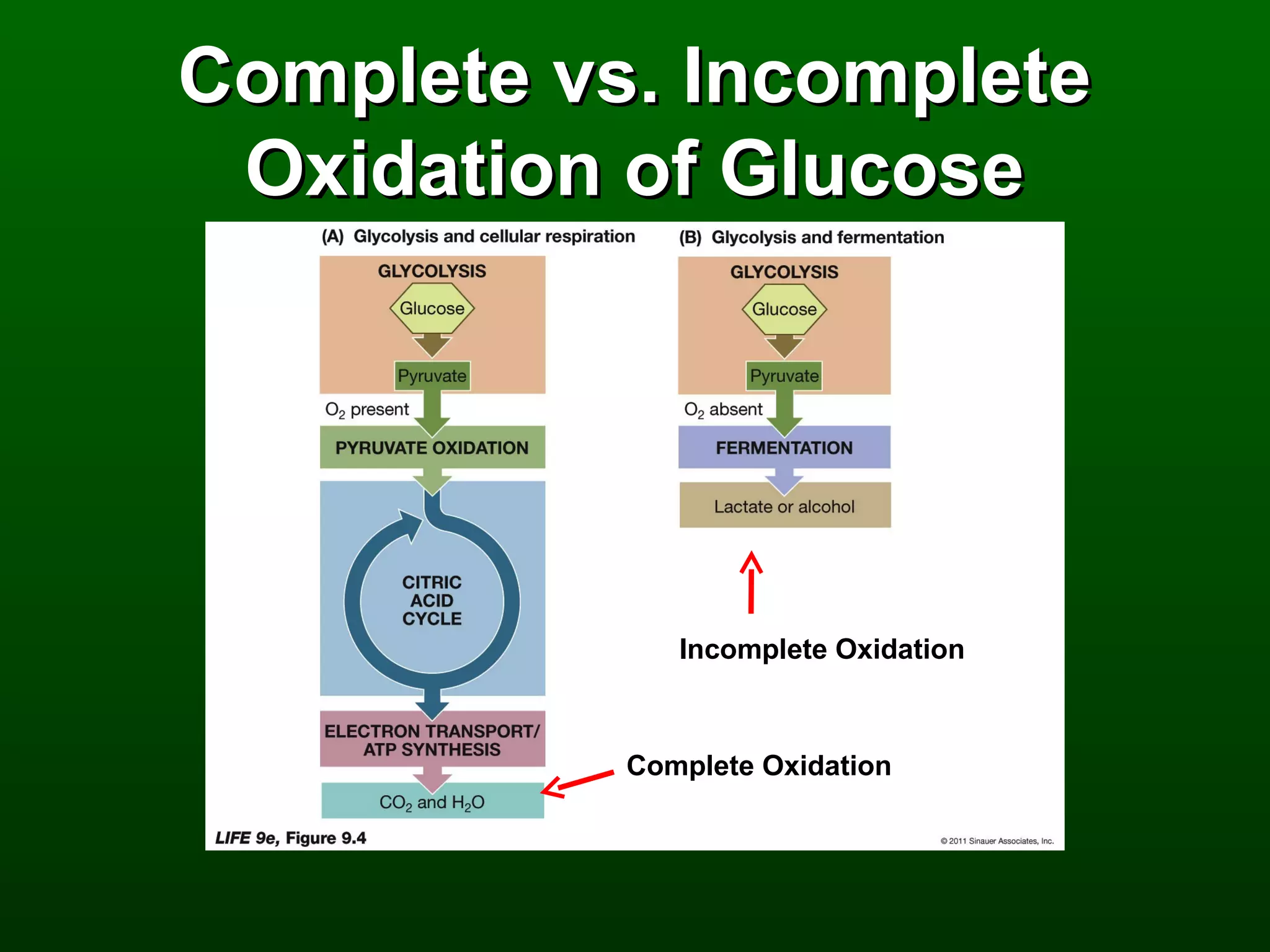
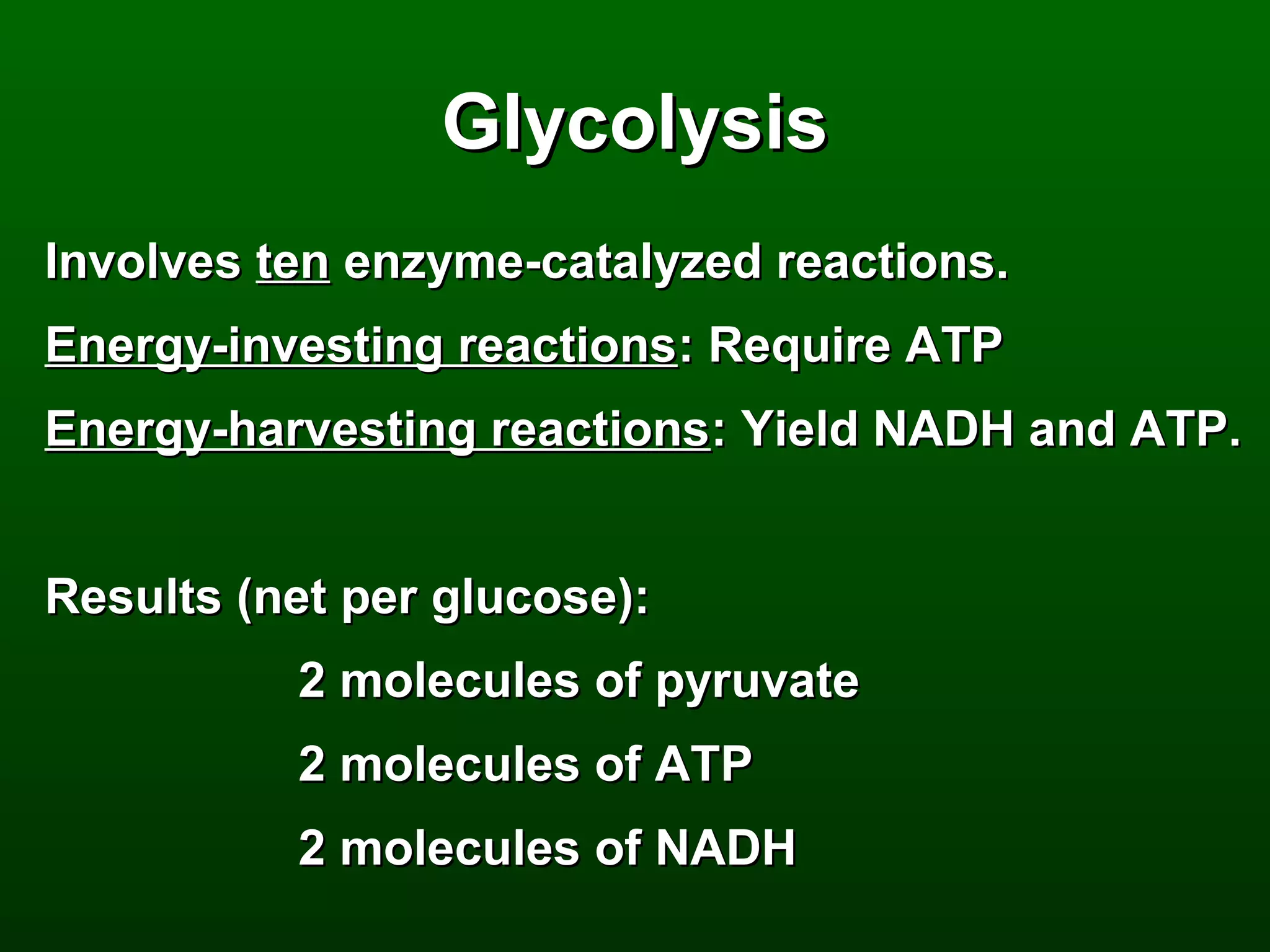
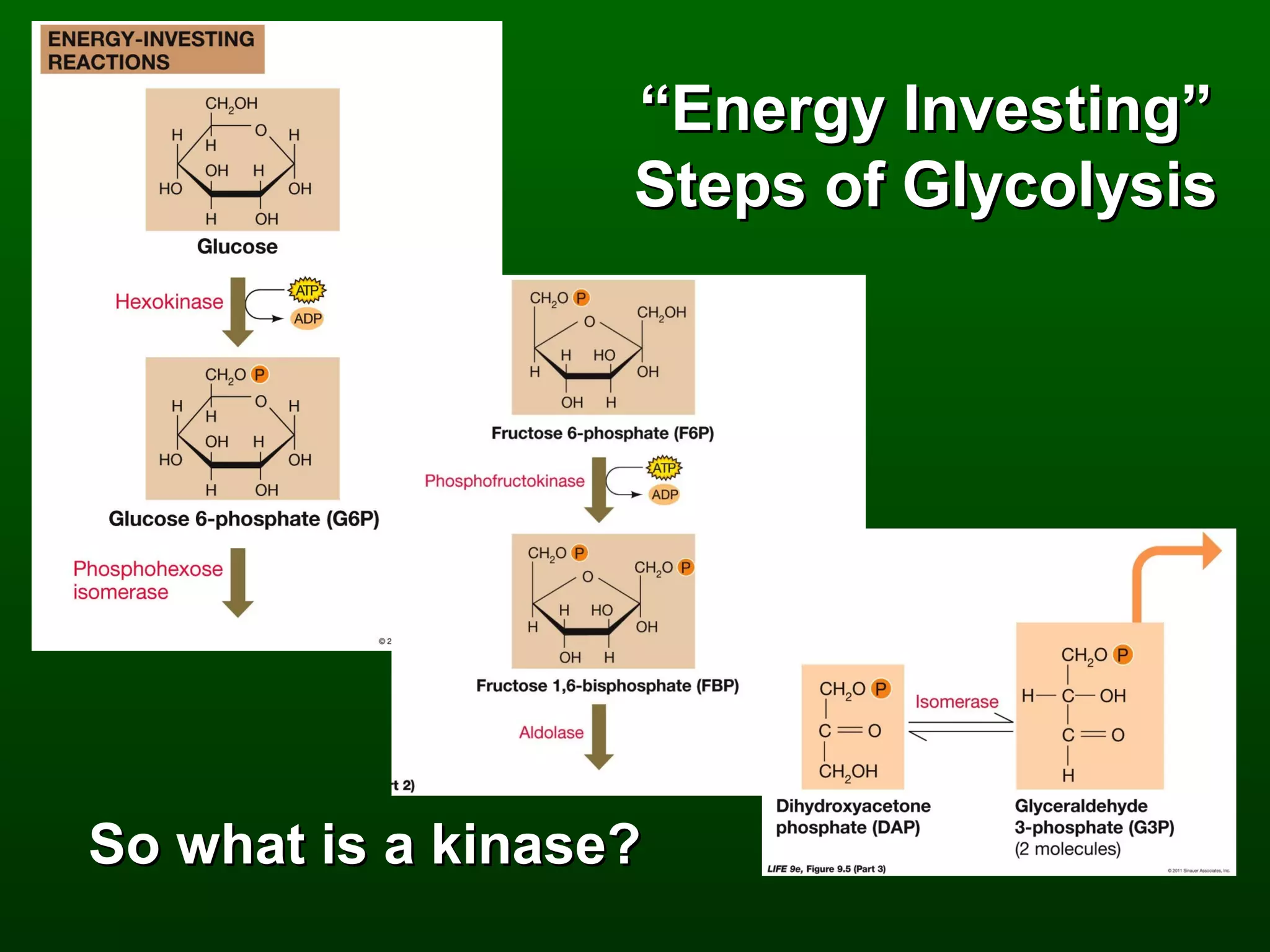
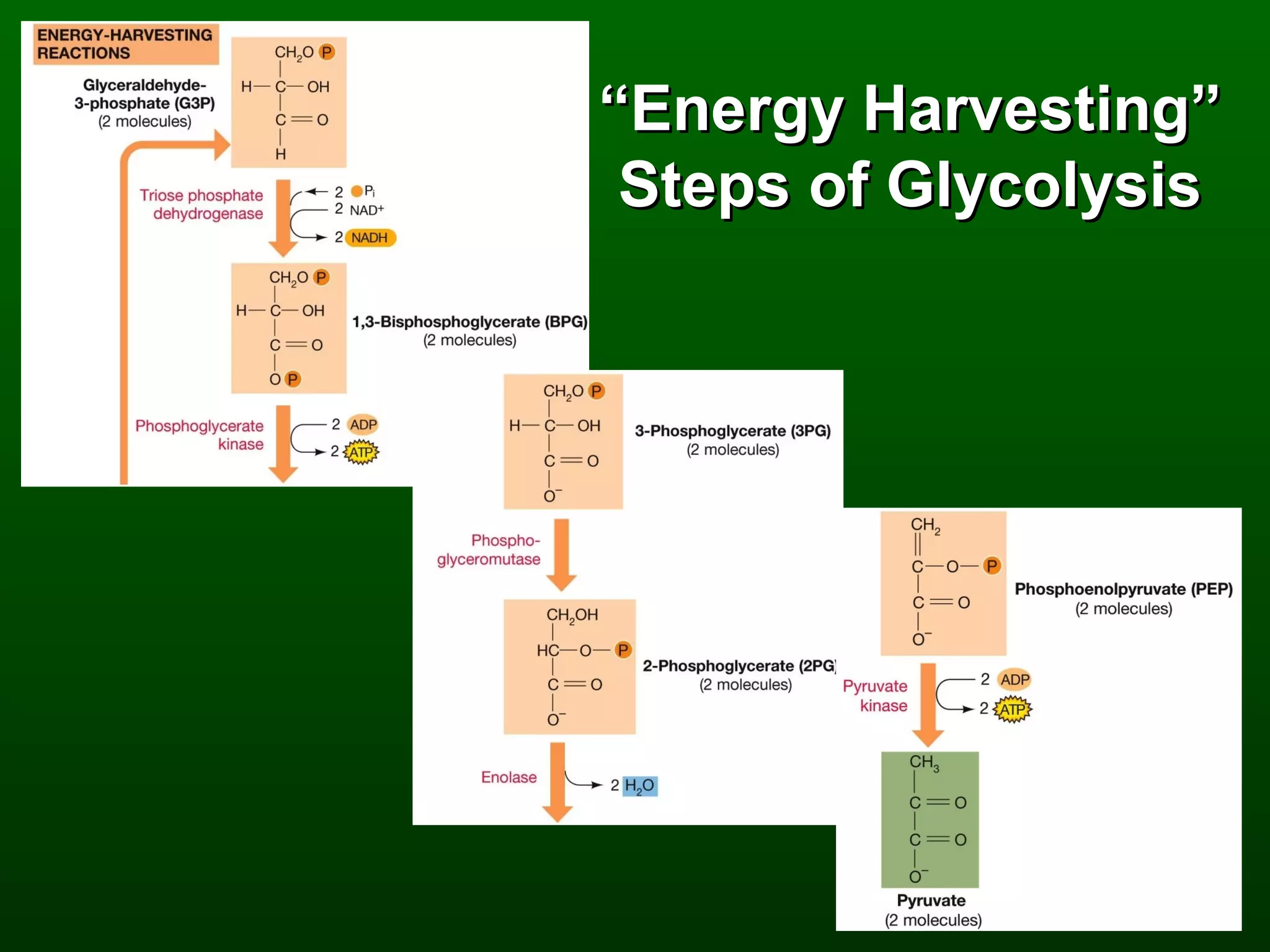
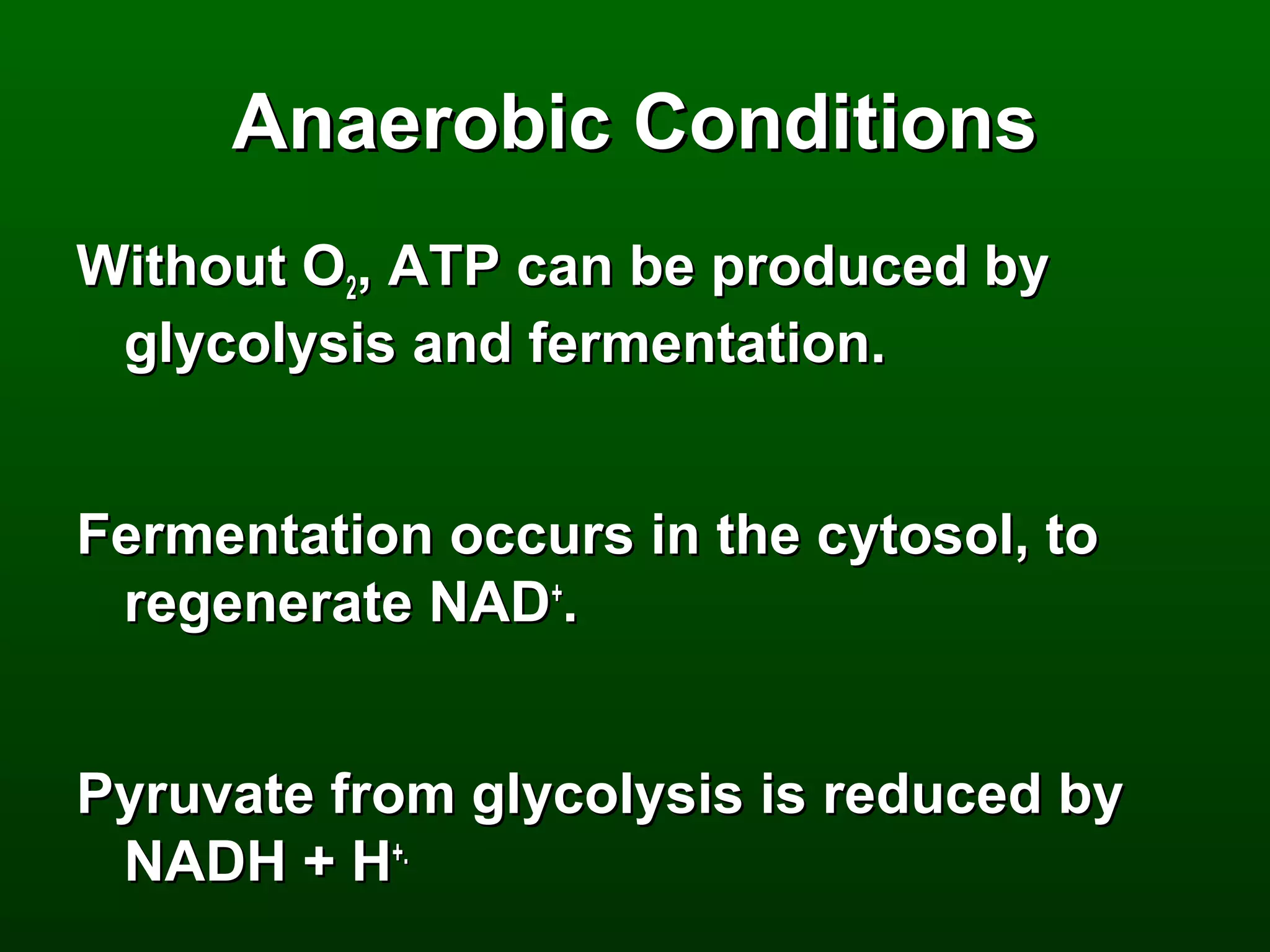
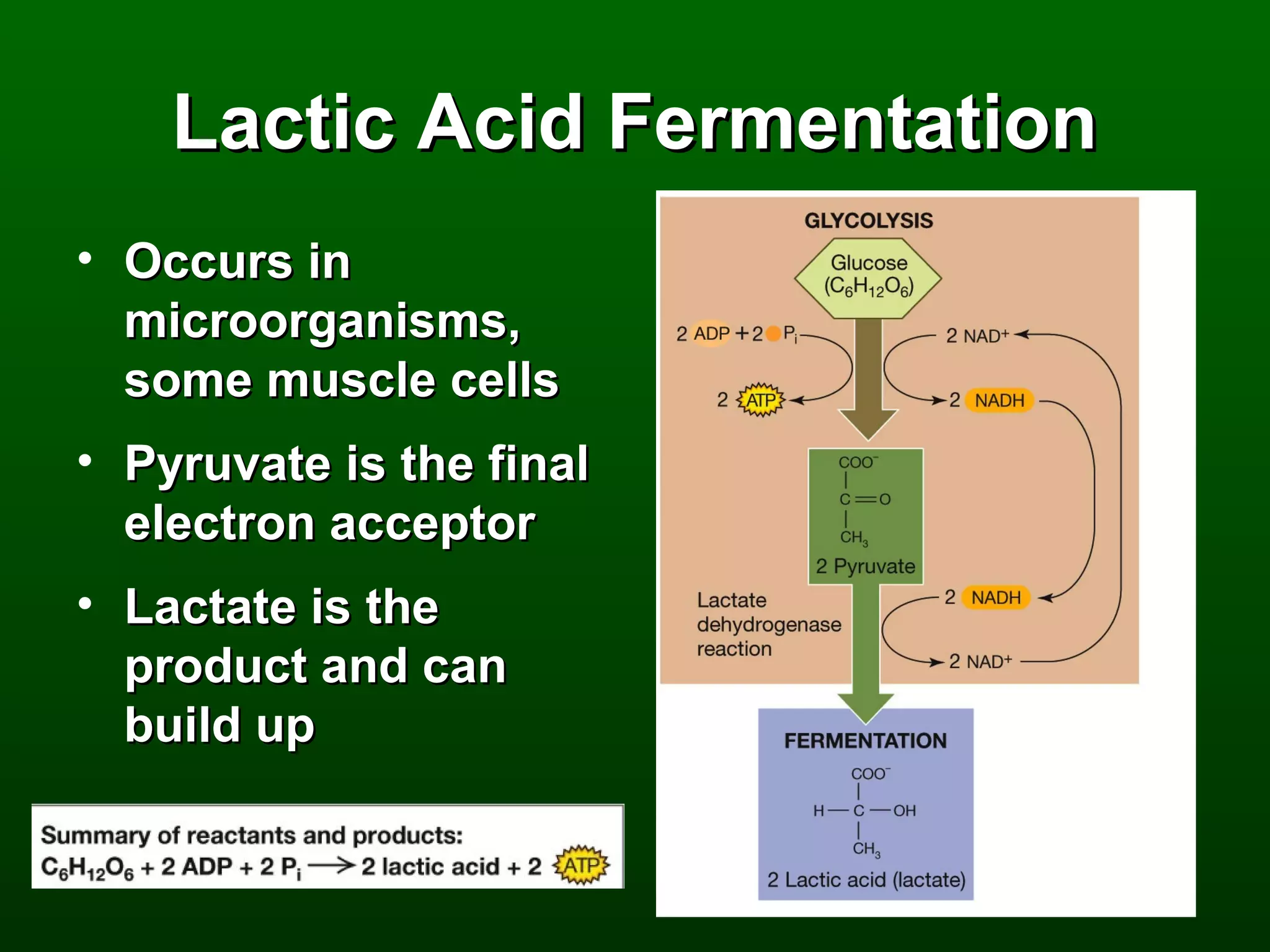
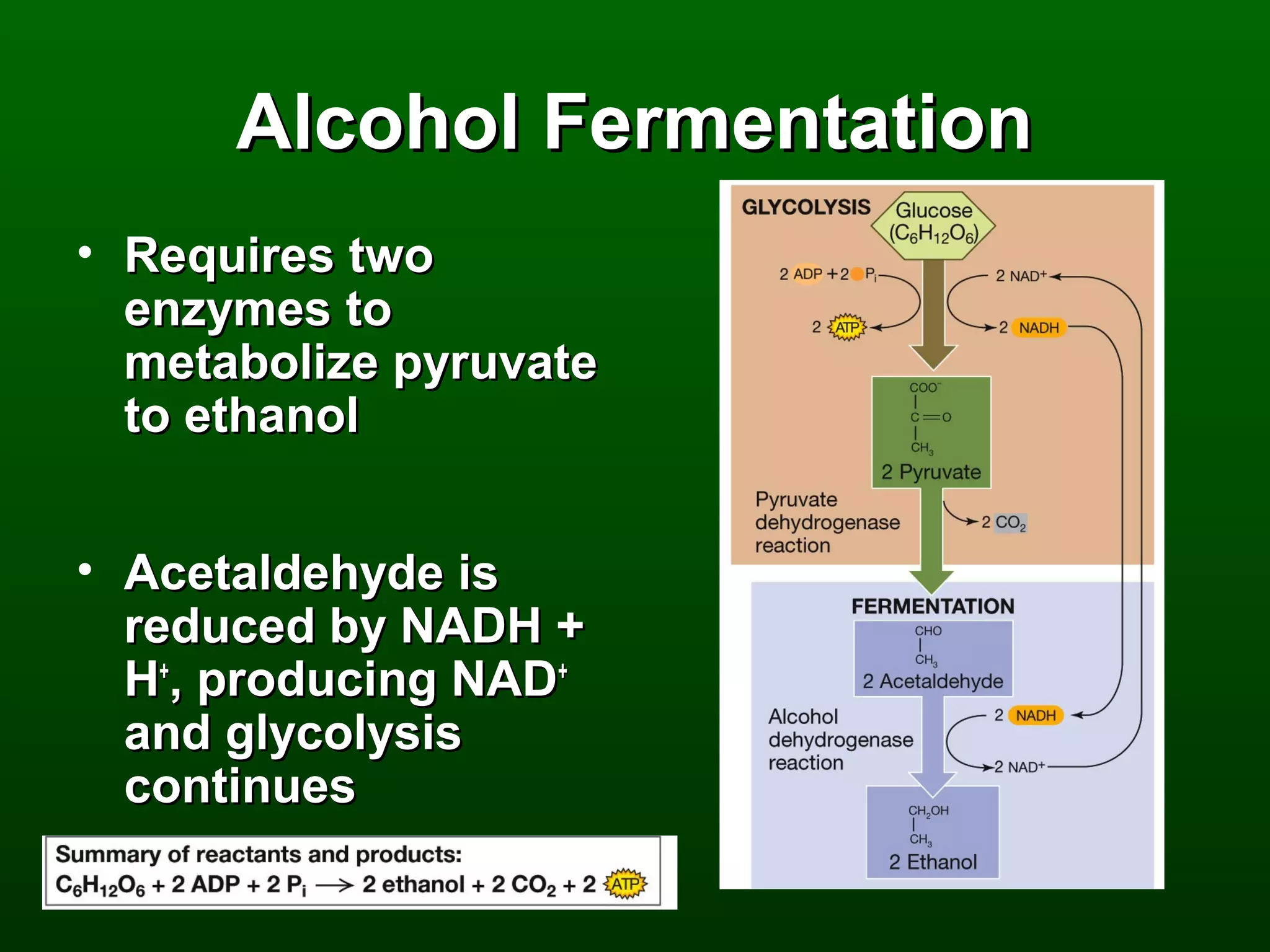
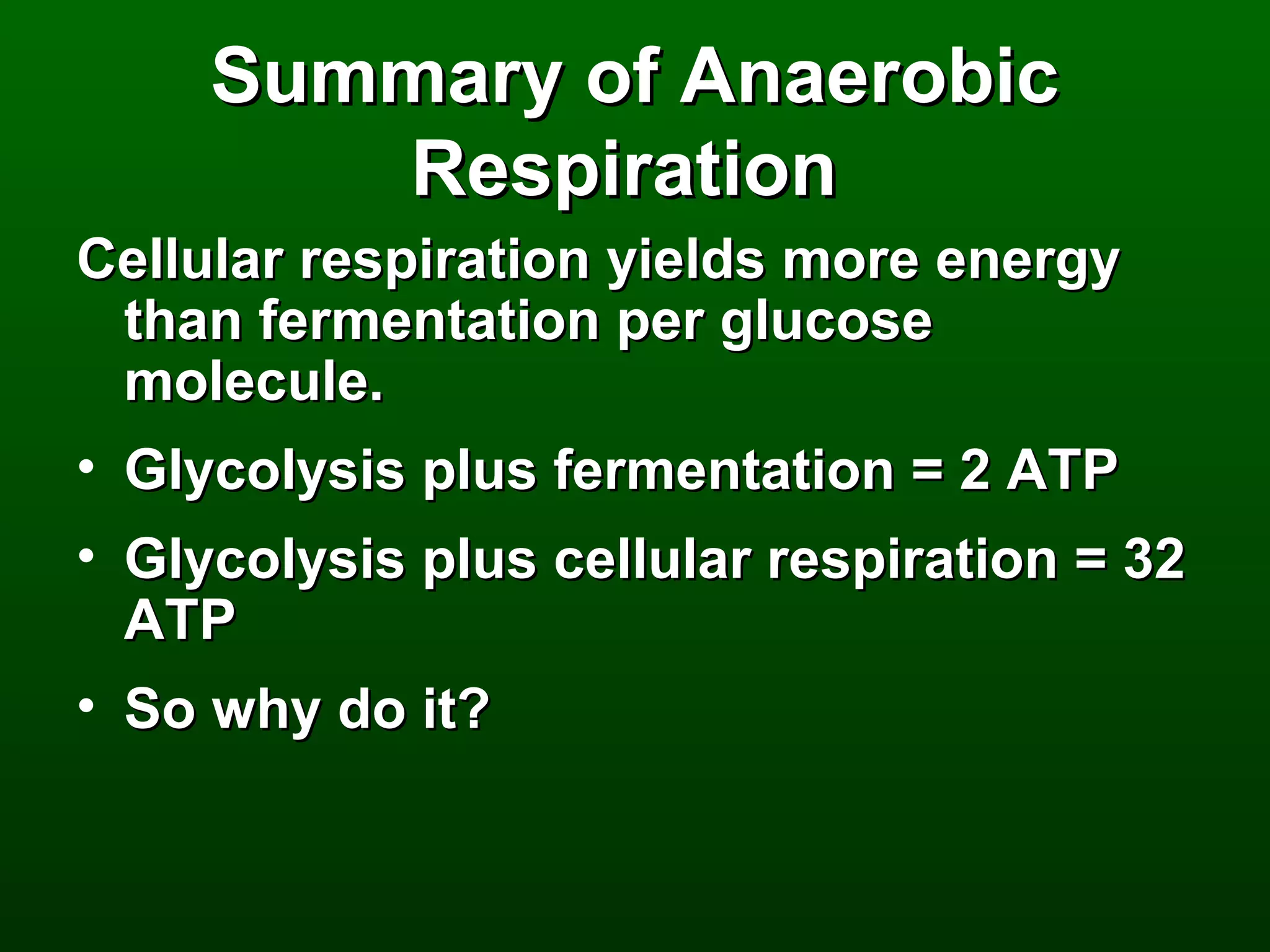
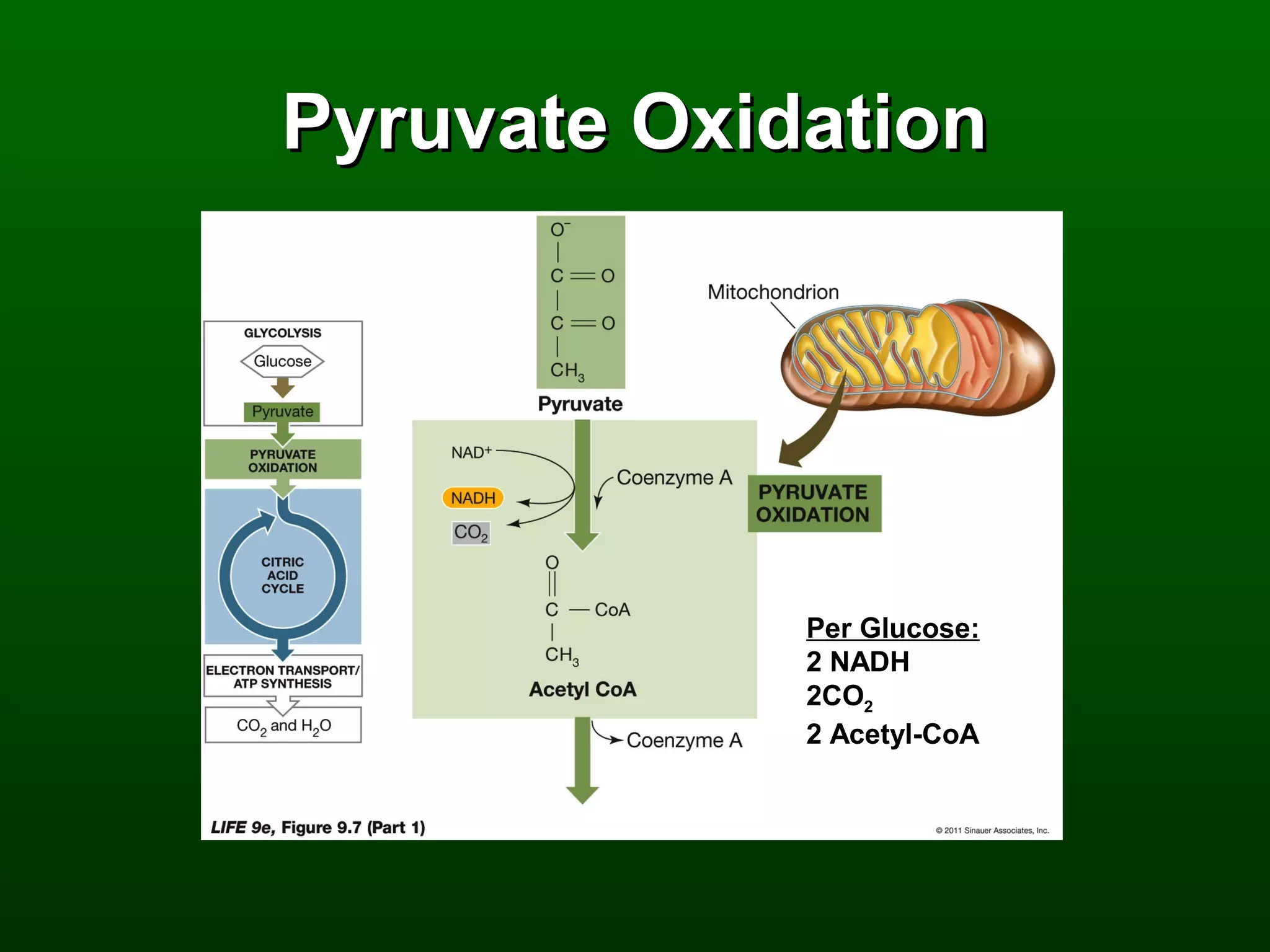
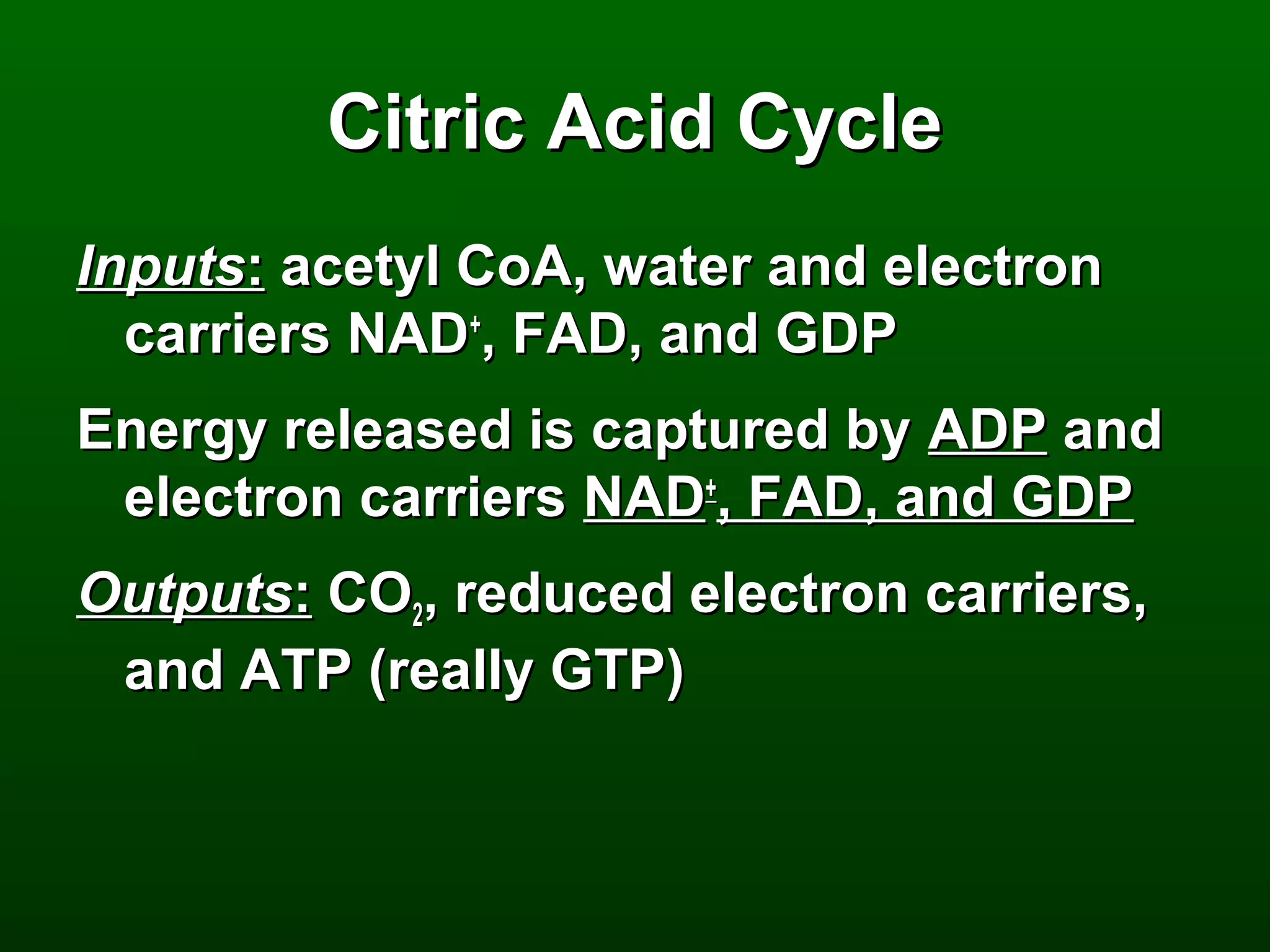
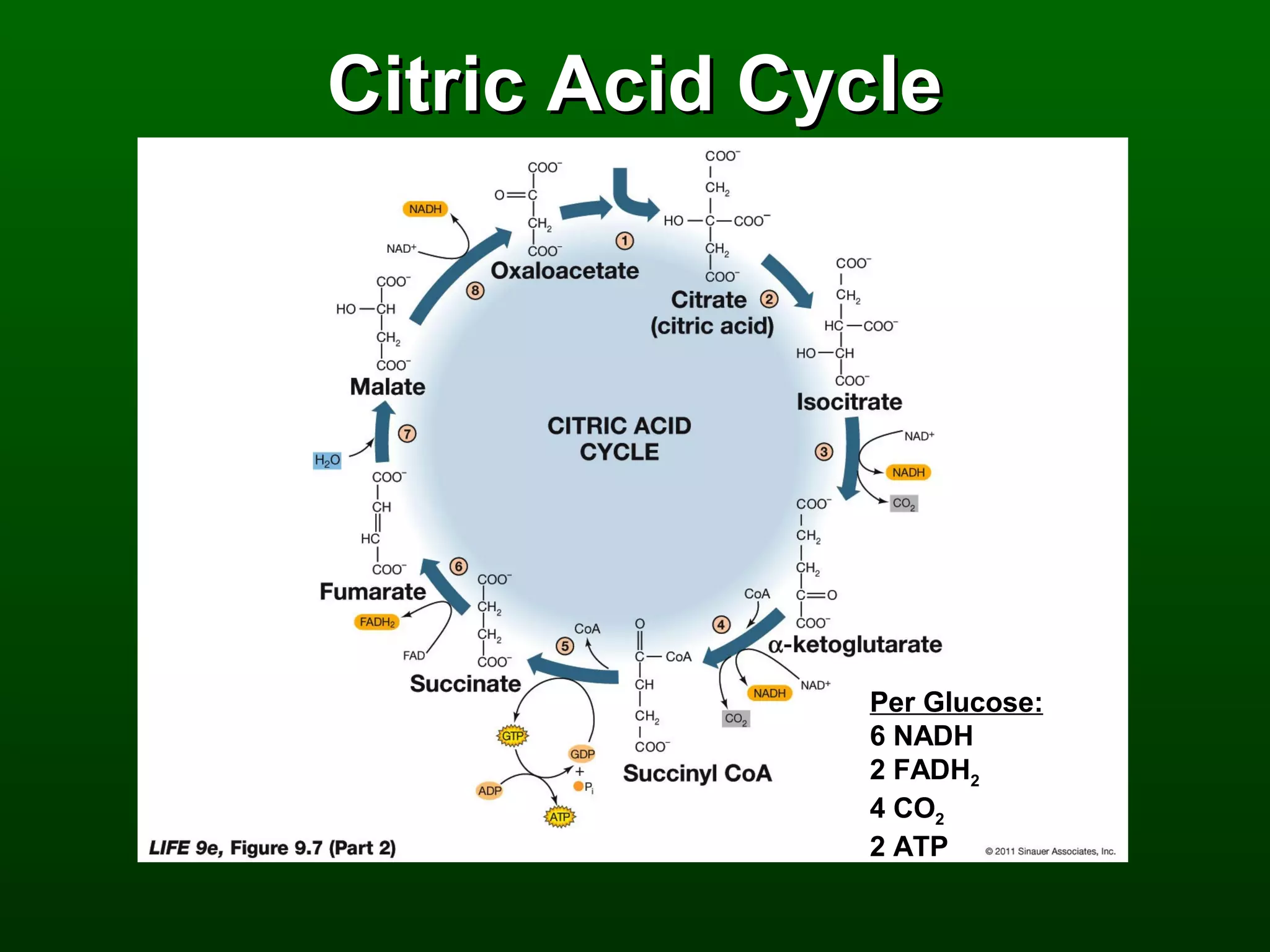
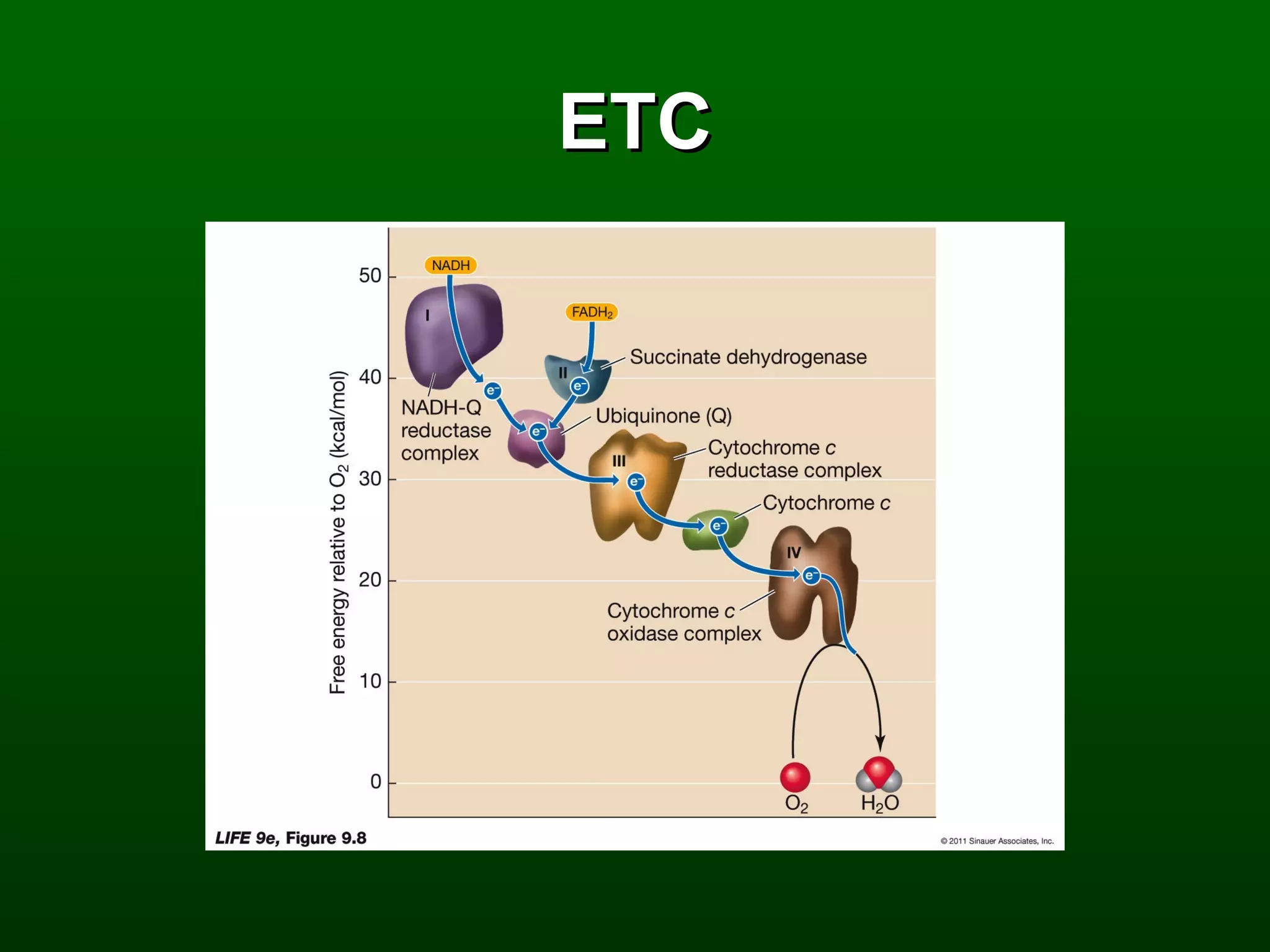
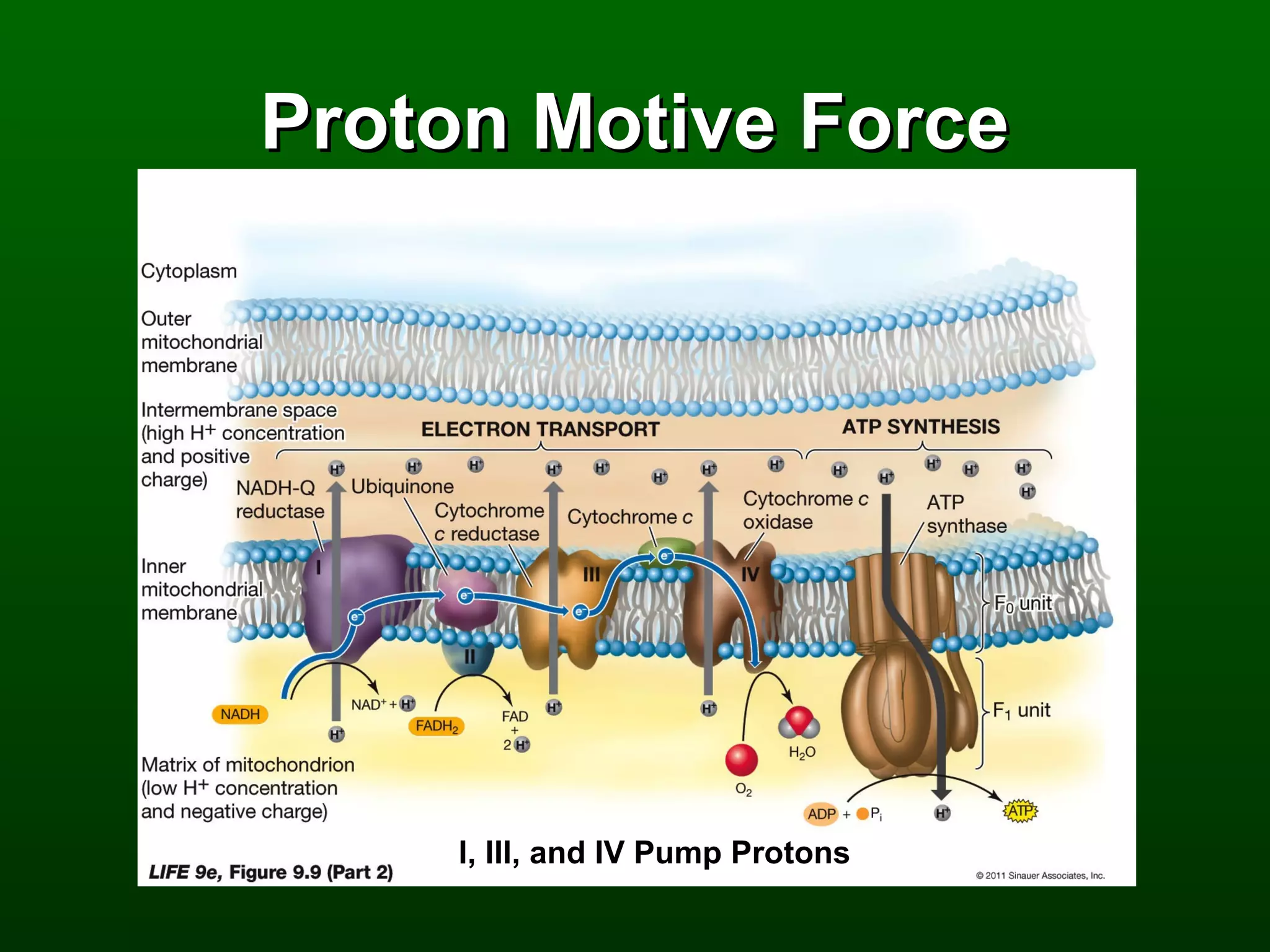
2) There are three main pathways: glycolysis, which converts glucose to pyruvate and generates a small amount of ATP; cellular respiration, which further breaks down pyruvate through the citric acid cycle and electron transport chain to generate much more ATP; and fermentation, which regenerates NAD+ without oxygen.
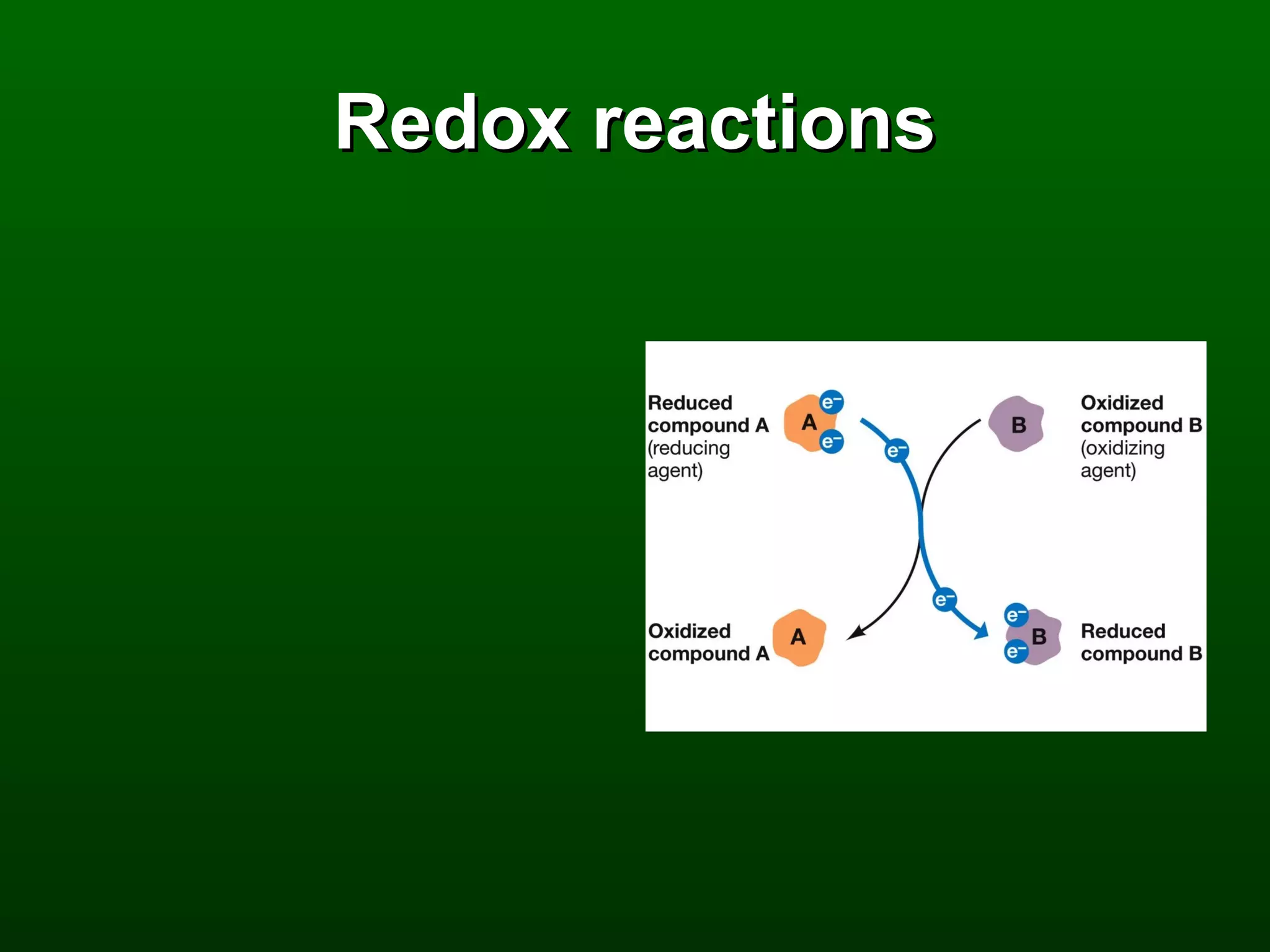
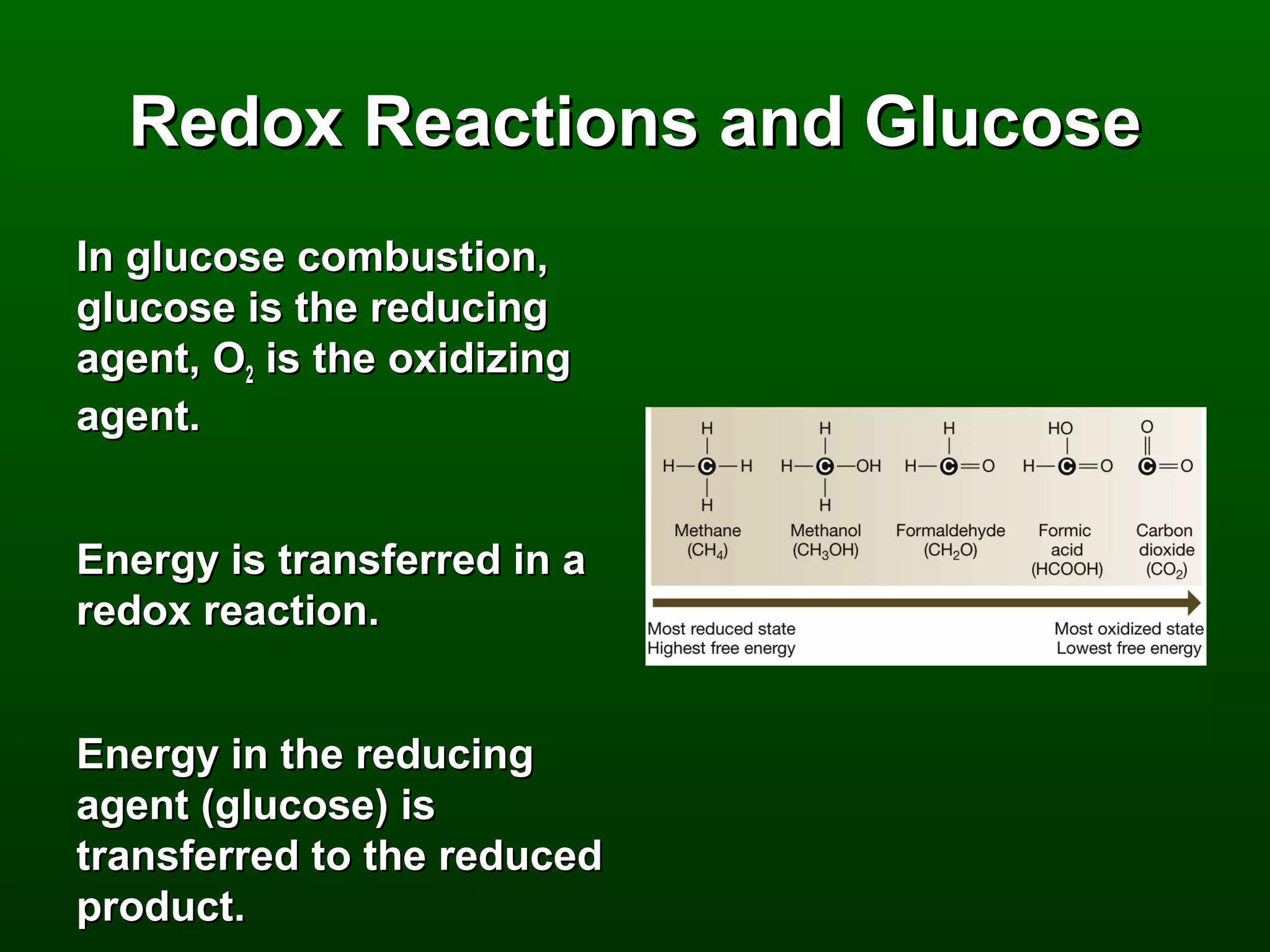
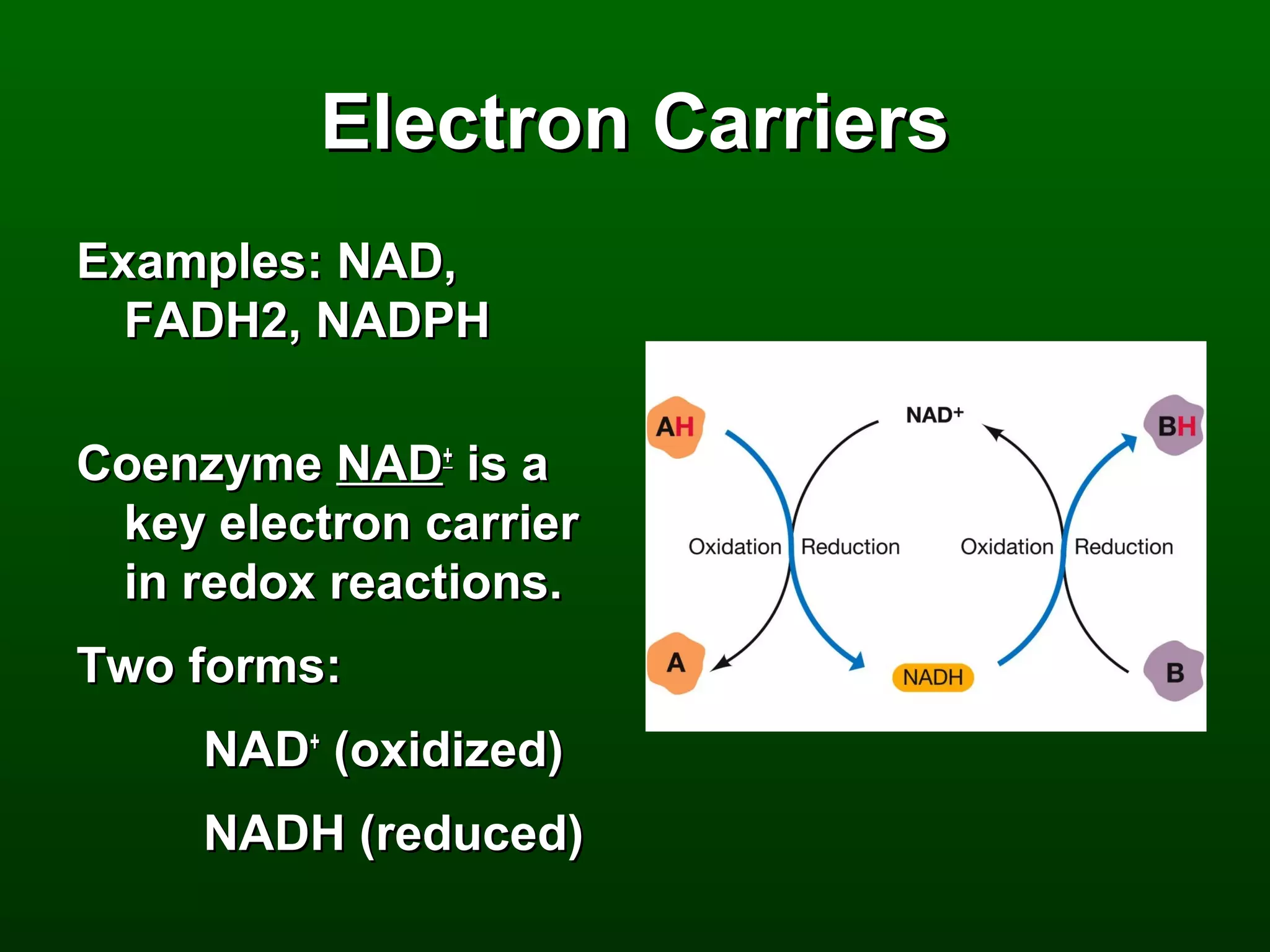
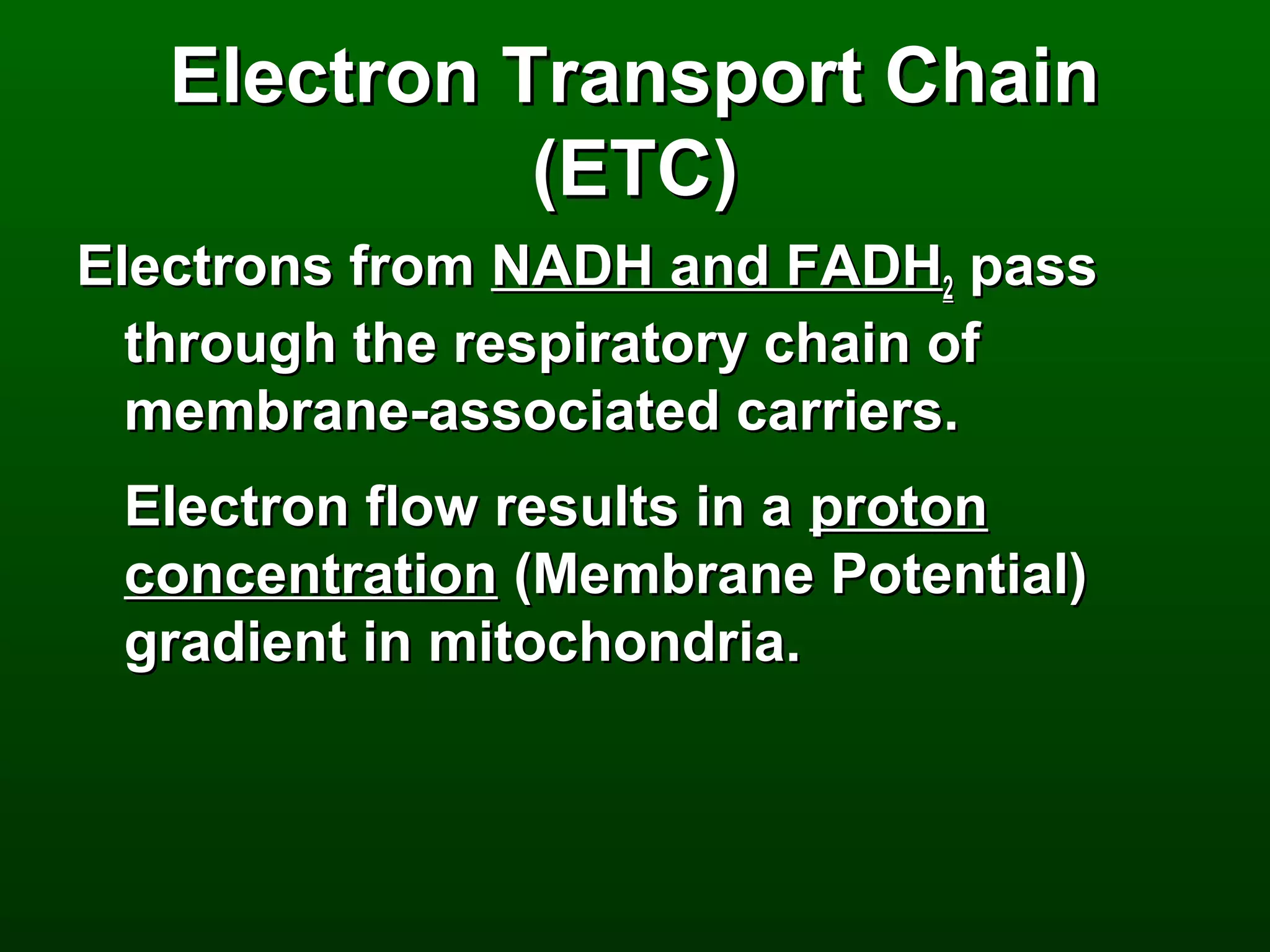
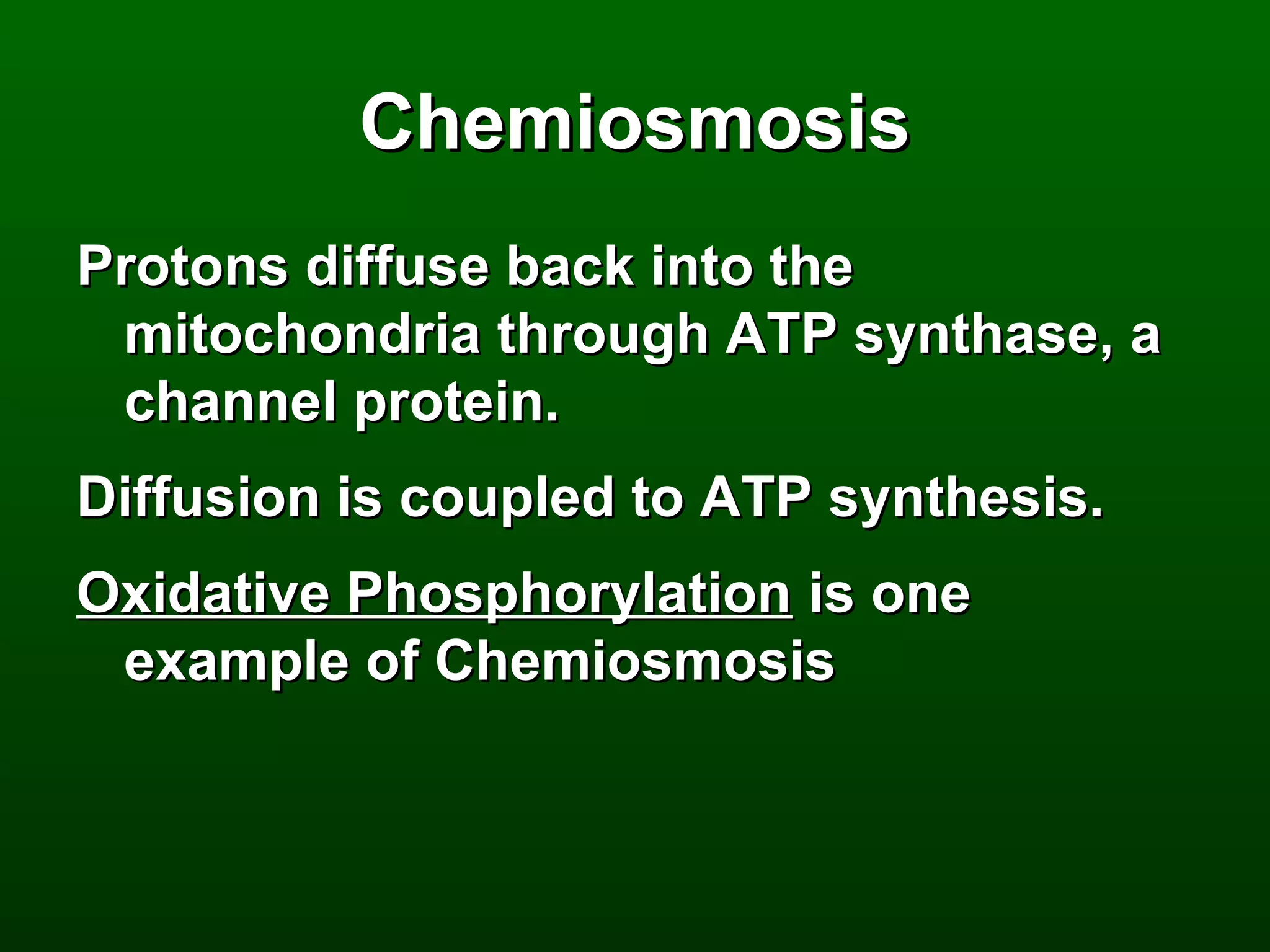
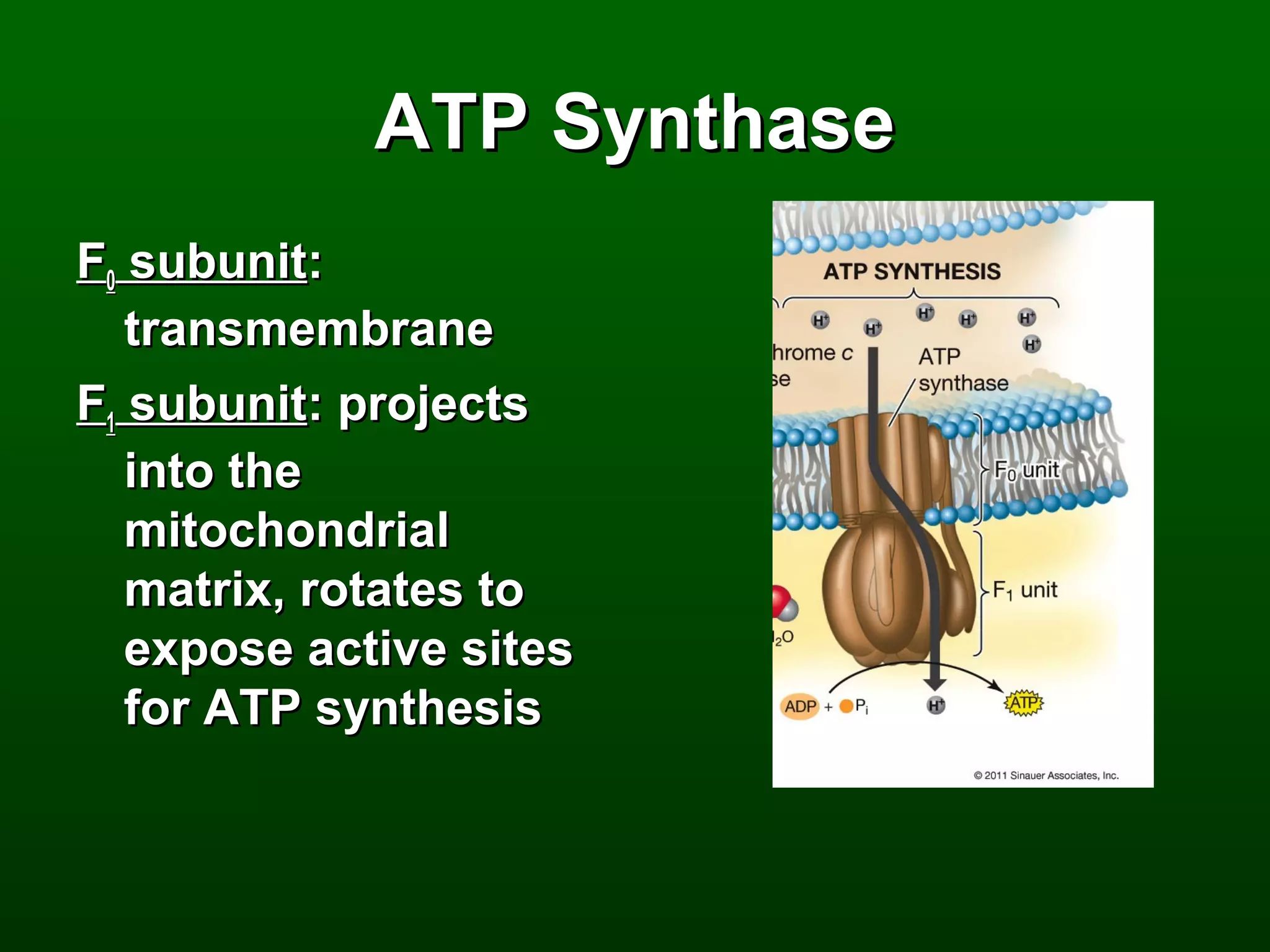
3) Through these pathways, the chemical energy from glucose is transferred to ATP via redox reactions and the proton gradient across the mitochondrial membrane during oxidative phosphorylation.