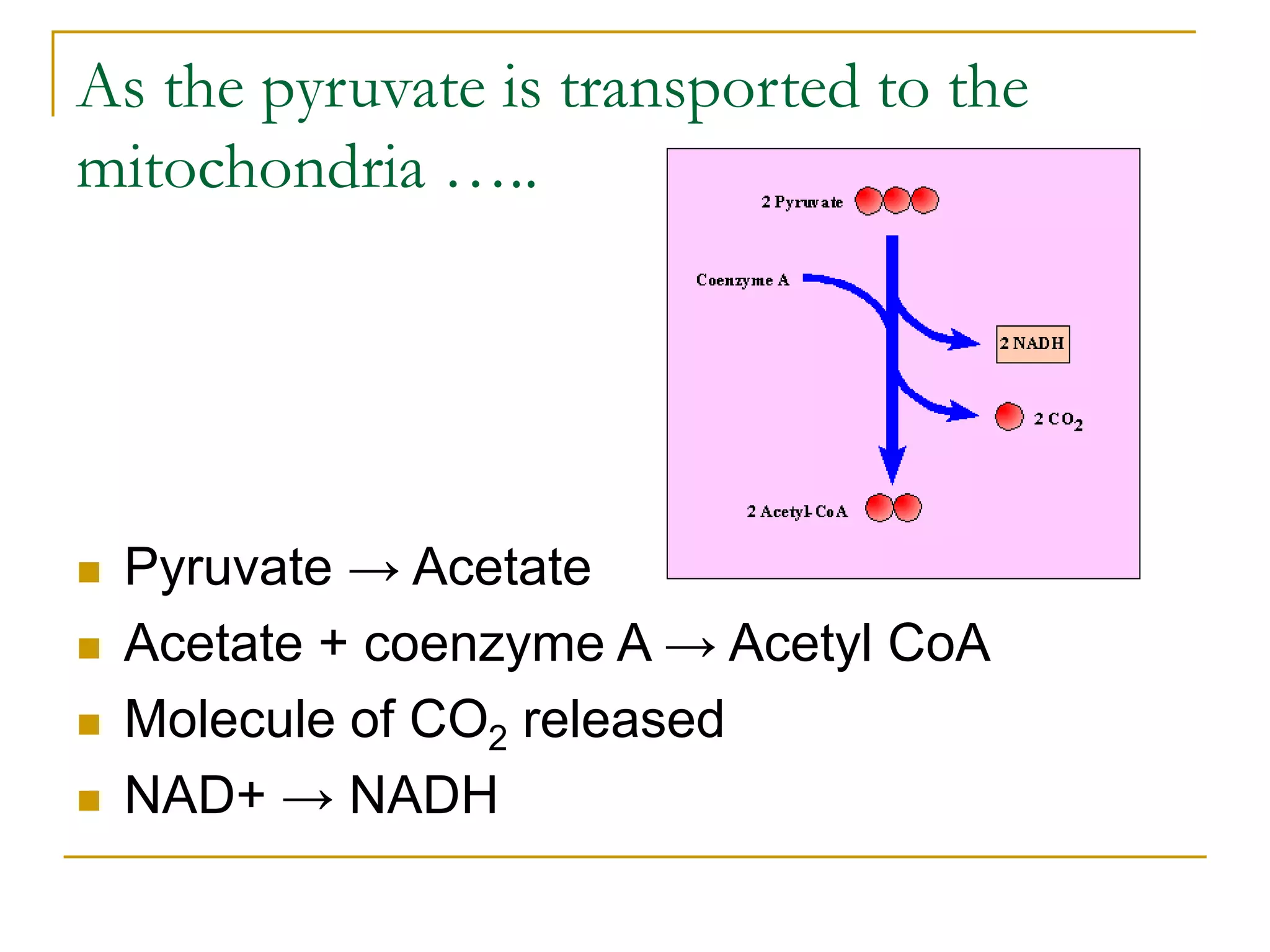
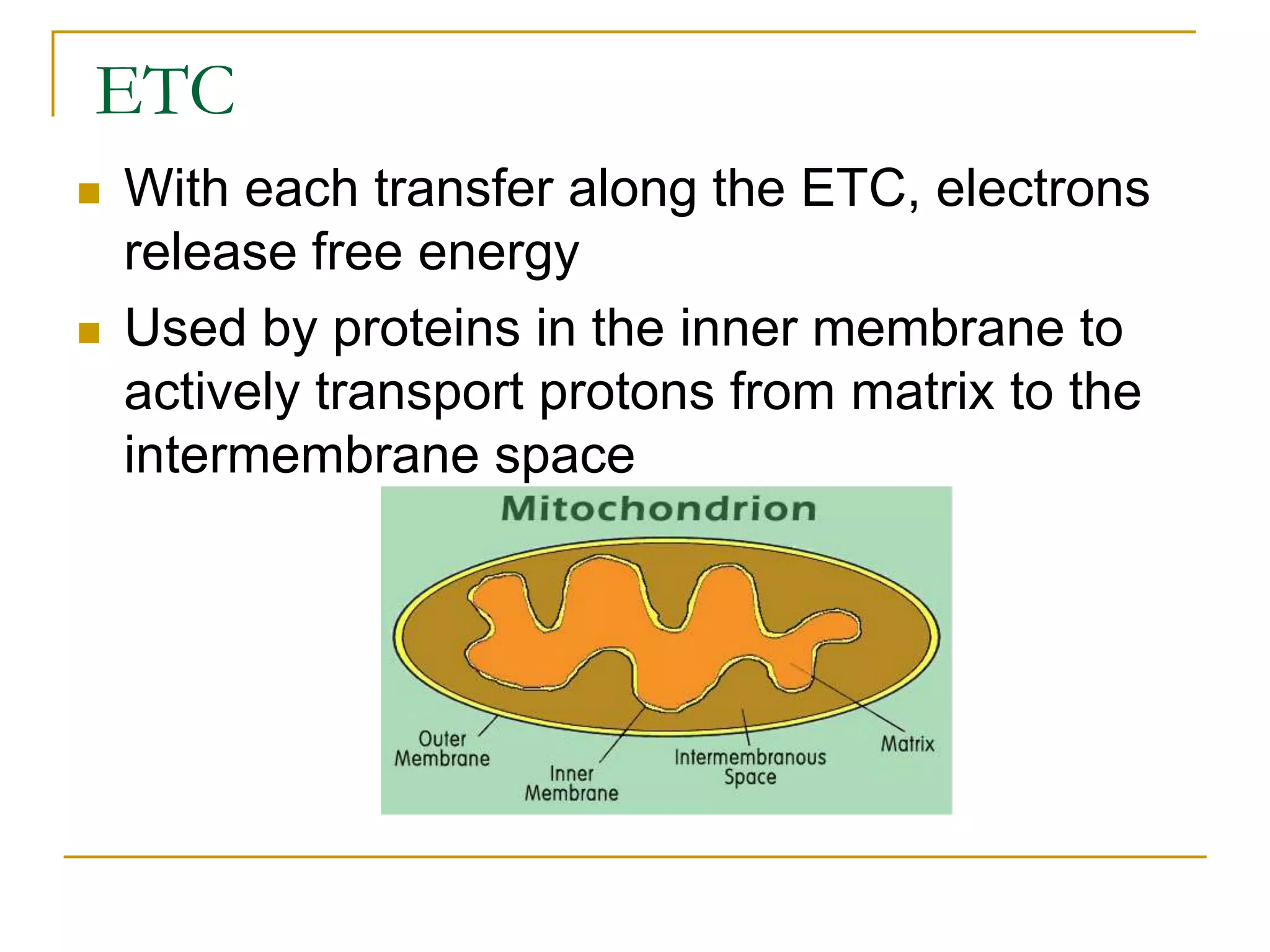
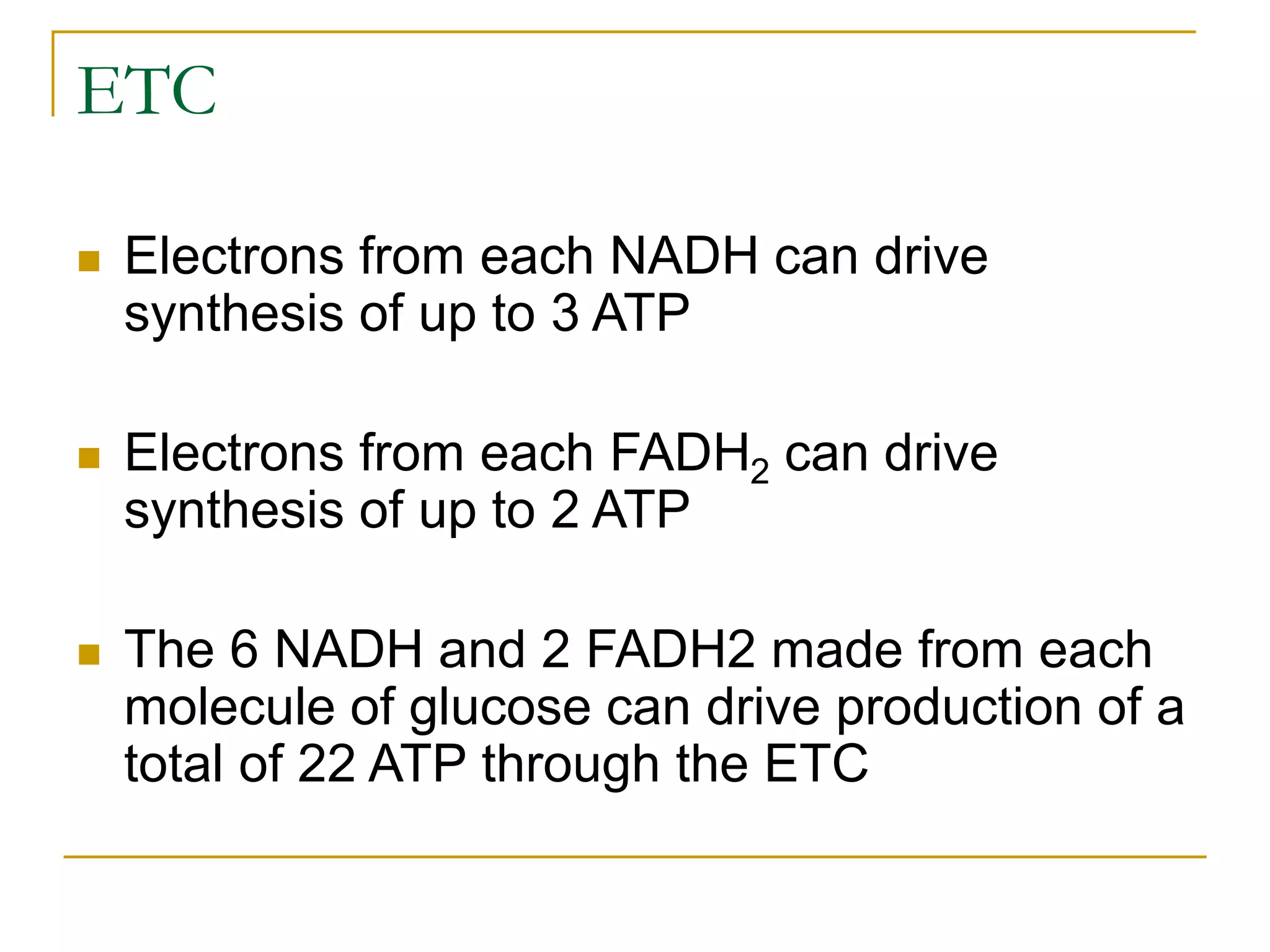
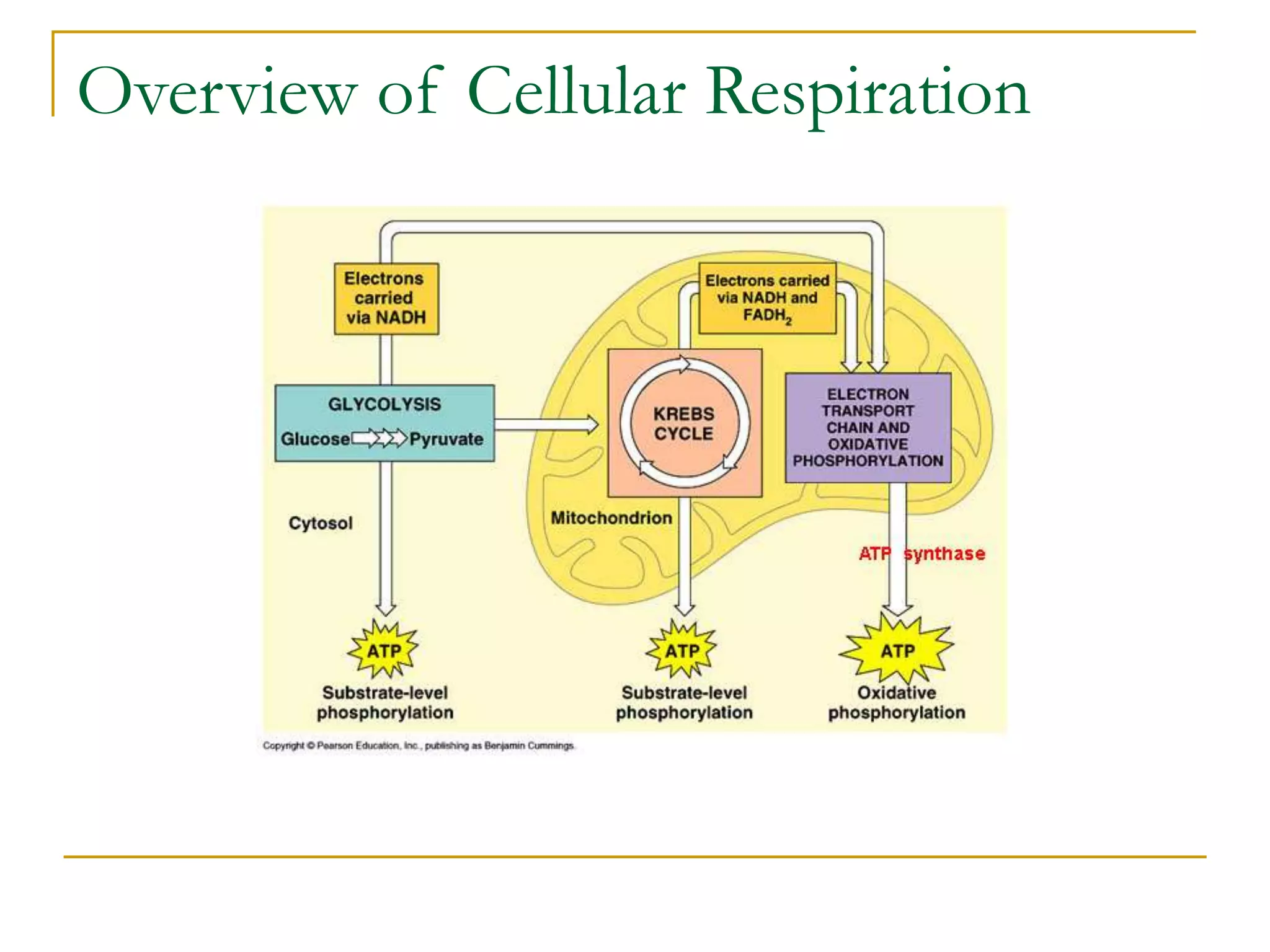
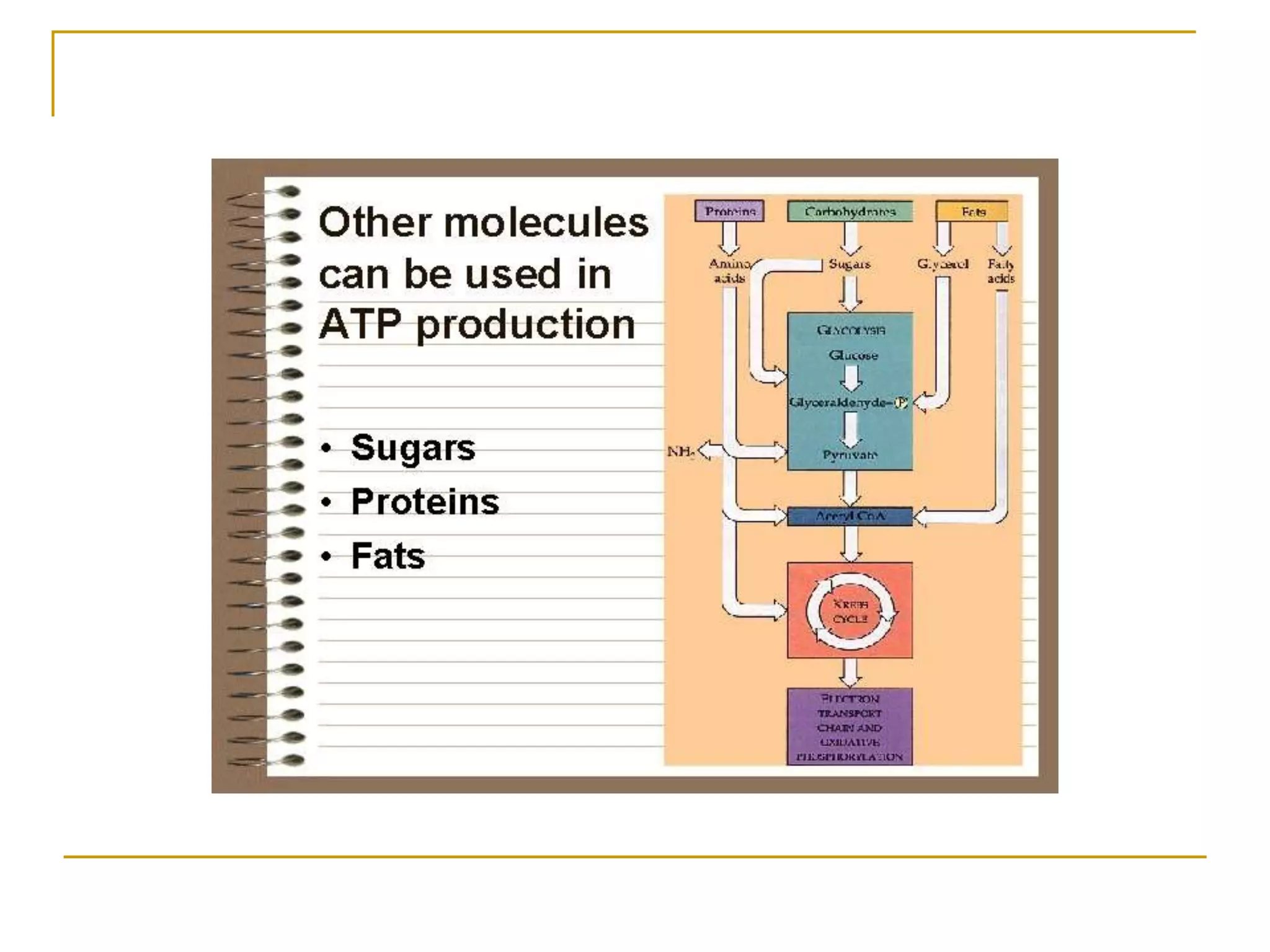
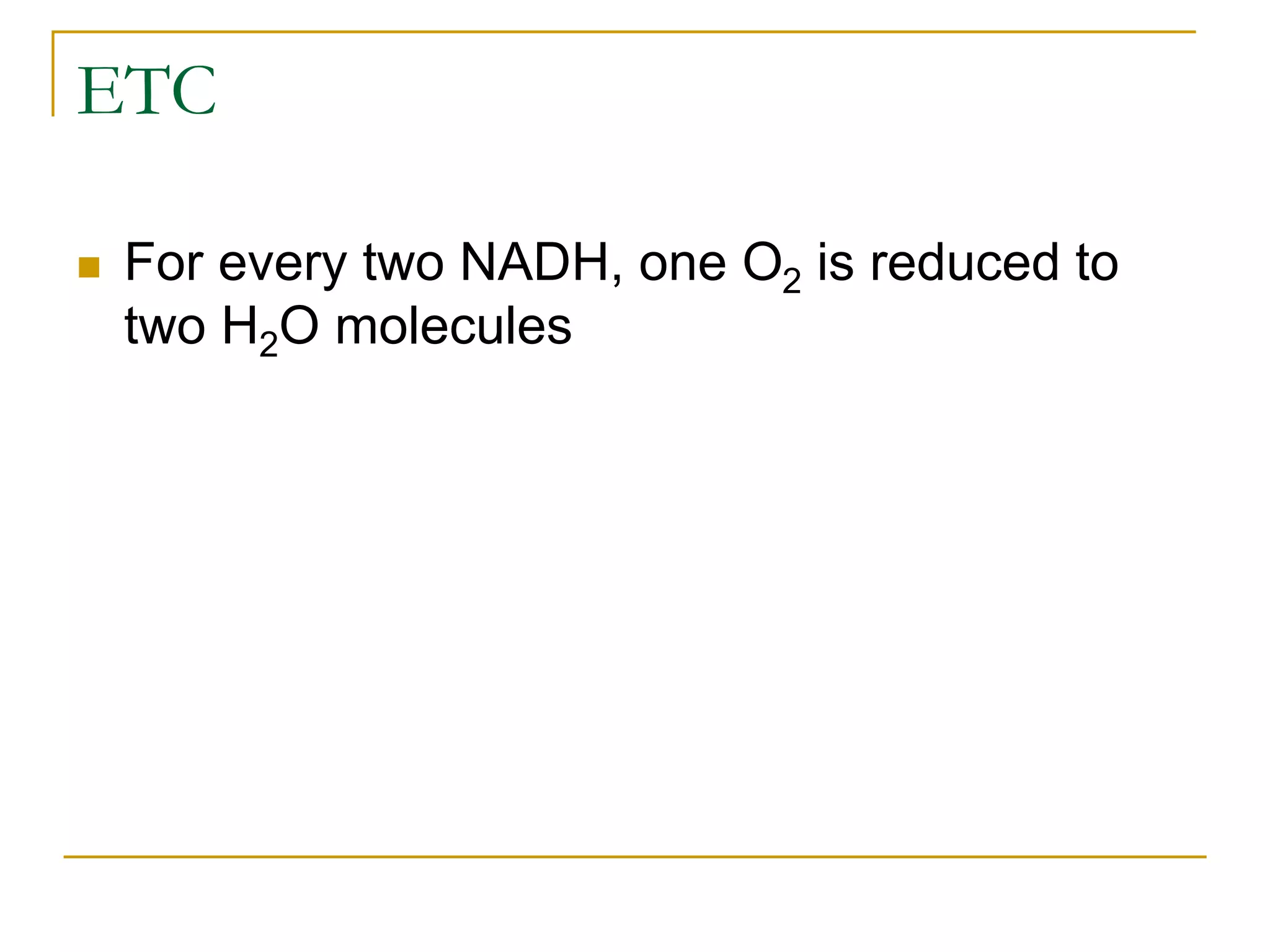Cellular respiration involves three main stages - glycolysis, the Krebs cycle, and the electron transport chain. Glycolysis breaks down glucose into pyruvate and produces a small amount of ATP. In the presence of oxygen, pyruvate enters the mitochondria and is further oxidized in the Krebs cycle, producing more ATP, NADH, and FADH2. These reduced coenzymes donate their electrons to the electron transport chain, where oxygen is the final electron acceptor. This powers ATP synthase to produce the majority of the cell's ATP through chemiosmosis. In total, the complete oxidation of one glucose molecule yields approximately 38 ATP.