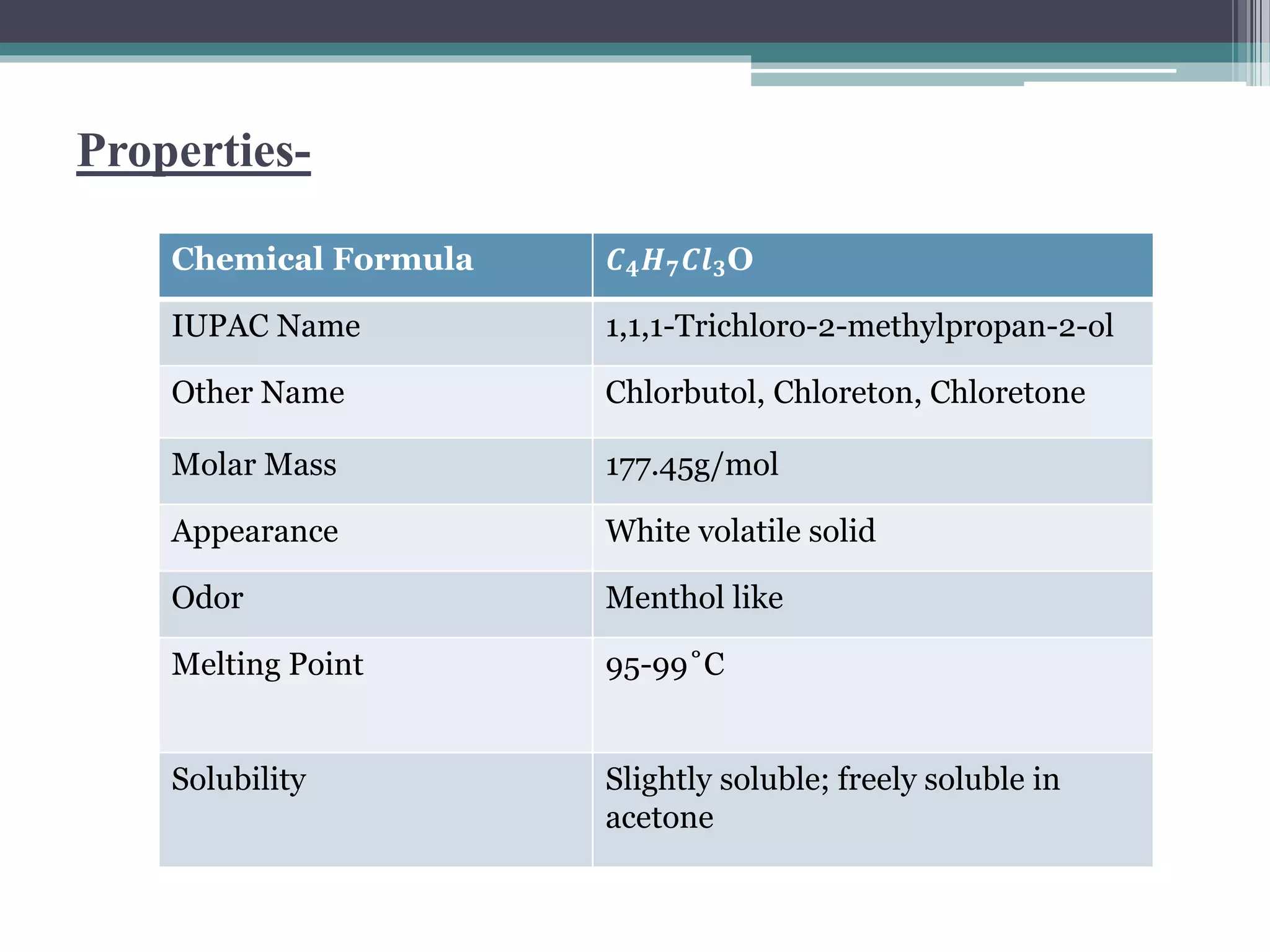
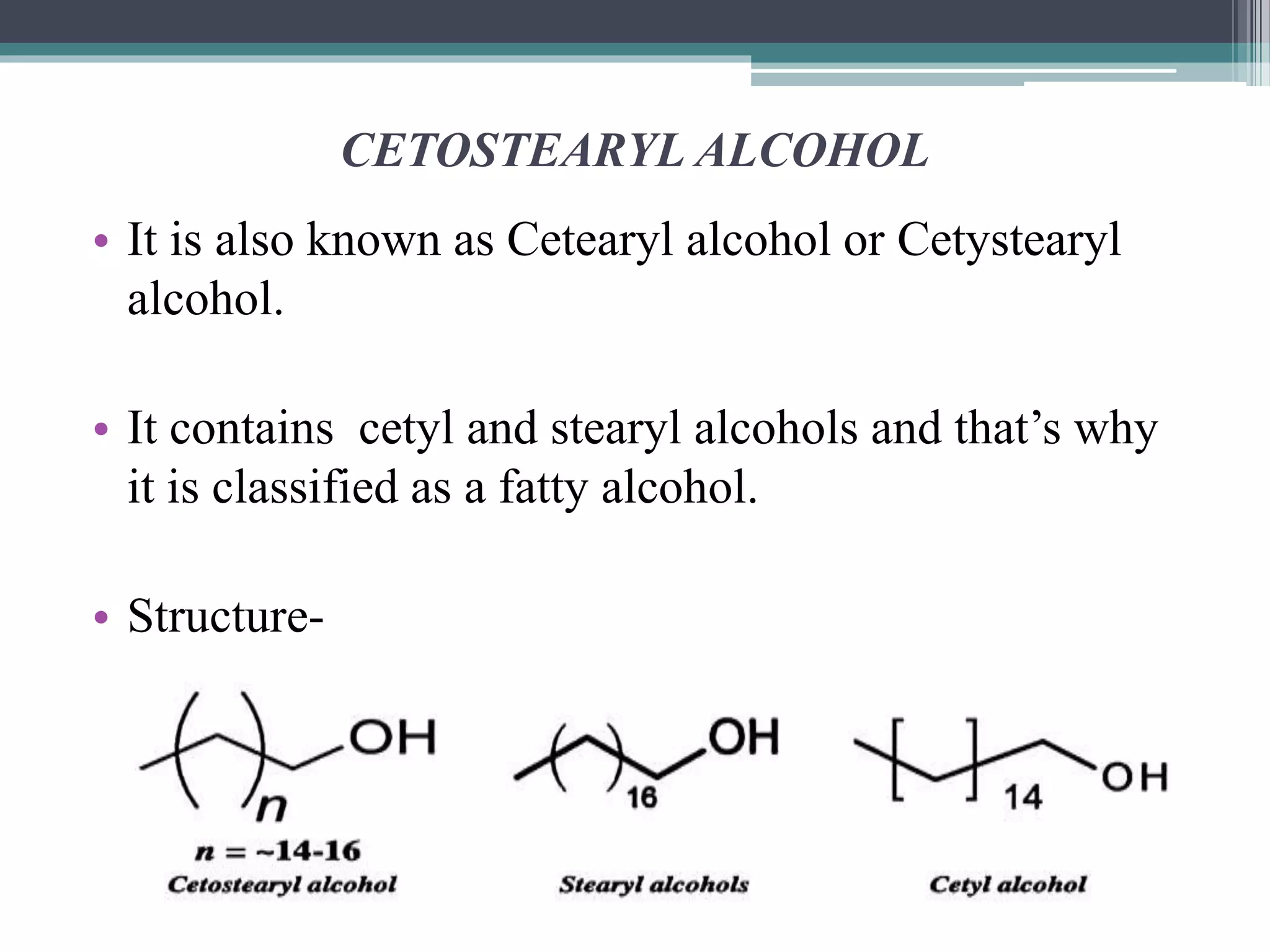
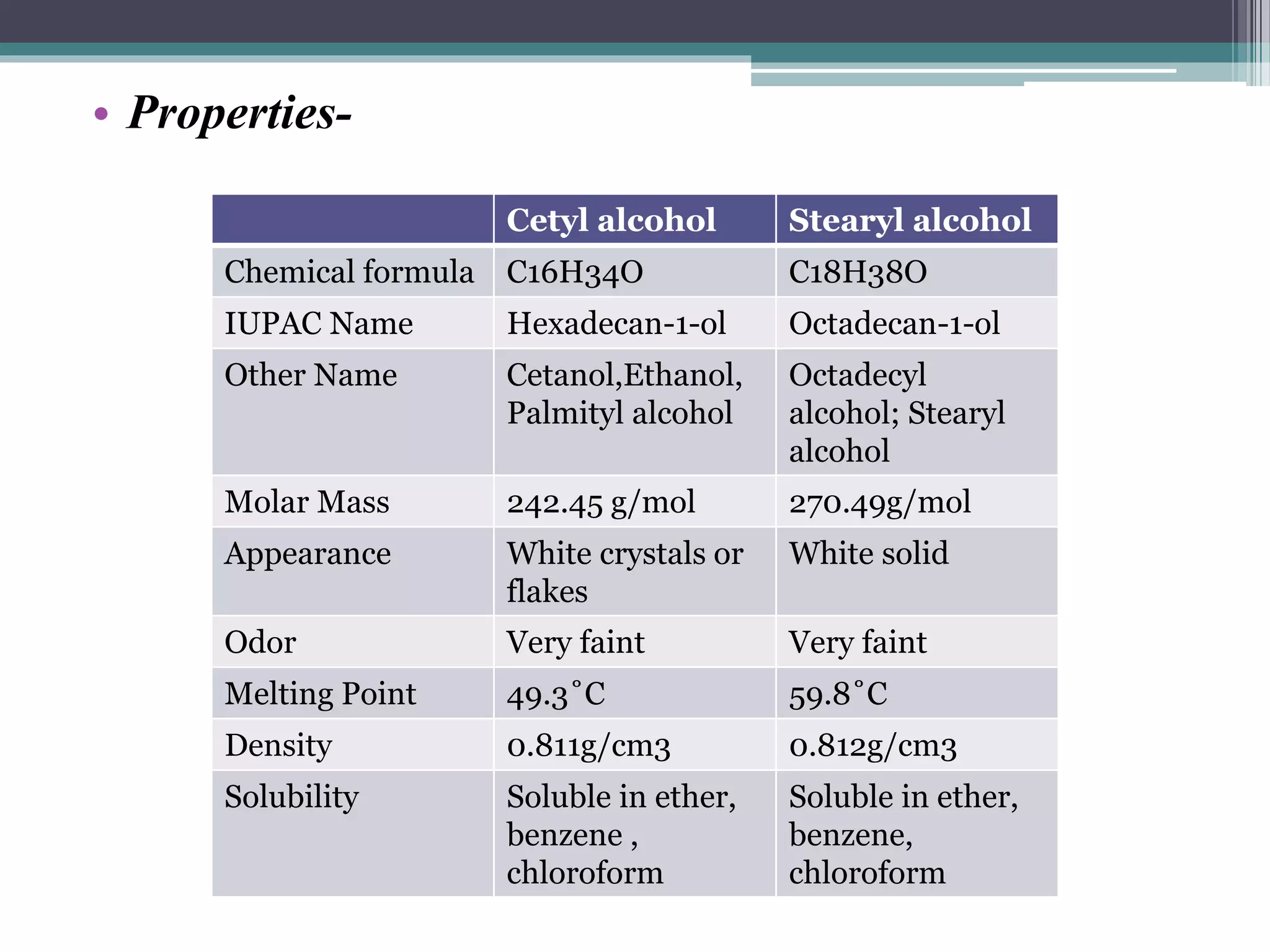
This document discusses various types of alcohols including their structures, properties, preparation methods and uses. It defines alcohols as organic compounds containing a hydroxyl functional group bonded to a carbon. Alcohols are classified based on the number of OH groups as monohydric, dihydric or trihydric. They are also classified based on the number of alkyl groups attached to the carbon as primary, secondary or tertiary alcohols. Common methods of preparing alcohols discussed are the hydrolysis of alkyl halides, reduction of carbonyl compounds, and hydration of alkenes. Specific alcohols described include ethanol, methanol, chlorobutanol, cetostearyl


![Classification of alcohols
Alcohols
[Based on no. of
OH group]
Monohydric alcohol-
Contains one OH
group
Eg- CH3CH2OH
Dihydric alcohol-
Contains 2 OH
group
Eg- CH2 OH
CH2 OH
Trihydric alcohol-
Contains 3 OH group
Eg- CH2 OH
CH2 OH
CH2 OH](https://image.slidesharecdn.com/alcohols-200618145503/75/Alcohols-3-2048.jpg)
![ALCOHOL
[No.ofalkylgroup]
PRIMARY
SECONDARY
TERTIARY](https://image.slidesharecdn.com/alcohols-200618145503/75/Alcohols-4-2048.jpg)




![• Complex metal hydrides- These are the best reducing
agents for the preparation of alcohol from aldehyde
and ketones in laboratory. Lithium aluminium
hydroxide [LiAlH4] and sodium borohydride
[NaBH4].
• Examples-
Reduction of Aldehydes-
O
R C H + 2[H] RCH2OH
Aldehyde Primary alcohol
Reducing agents- H2/Pt, NaBH4 or LiAlH4](https://image.slidesharecdn.com/alcohols-200618145503/75/Alcohols-9-2048.jpg)
![• Reduction of Ketones-
O H
R C R + 2[H] R C R
Ketone OH
Secondary alcohol
Reducing agents- H2/Pt, NaBH4 or LiAlH4
• Reduction of Carboxylic acids-
O
R C OH + 4[H] RCH4OH + H2O
Carboxylic acid
Reducing agents- B2H6; LiAlH4](https://image.slidesharecdn.com/alcohols-200618145503/75/Alcohols-10-2048.jpg)
![• Reduction of Carboxylic esters-
O
R C OR’ + 4[H] RCH2OH + R’OH
Carboxylic ester Mixtures
Note-
LiAlH4, does not reduce C=C so it is used for the
preparation of unsaturated alcohols from unsaturated
aldehyde and ketones.
Question- Which reducing agent is used to make unsaturated
alcohols and why?](https://image.slidesharecdn.com/alcohols-200618145503/75/Alcohols-11-2048.jpg)





![• Ceric ammonium nitrate test- Alcohol react with cerric
ammonium nitrate to form a red coloured alkoxy
cerium(IV) compound.
2R-OH+ [NH4]2Ce(NO3)6 ( ROH)2Ce(NO3)4 +2NH4NO3
Alcohol Ceric ammonium Alkoxy cerium(IV)
nitrate compound
[Pink or Red colour]
Example-
2CH3-OH+ [NH4]2Ce(NO3)6 (CH3OH)2Ce(NO3)4+2NH4NO3
Methanol Ceric ammonium Methoxy cerium(IV)
nitrate compound
[Pink or Red colour]](https://image.slidesharecdn.com/alcohols-200618145503/75/Alcohols-17-2048.jpg)

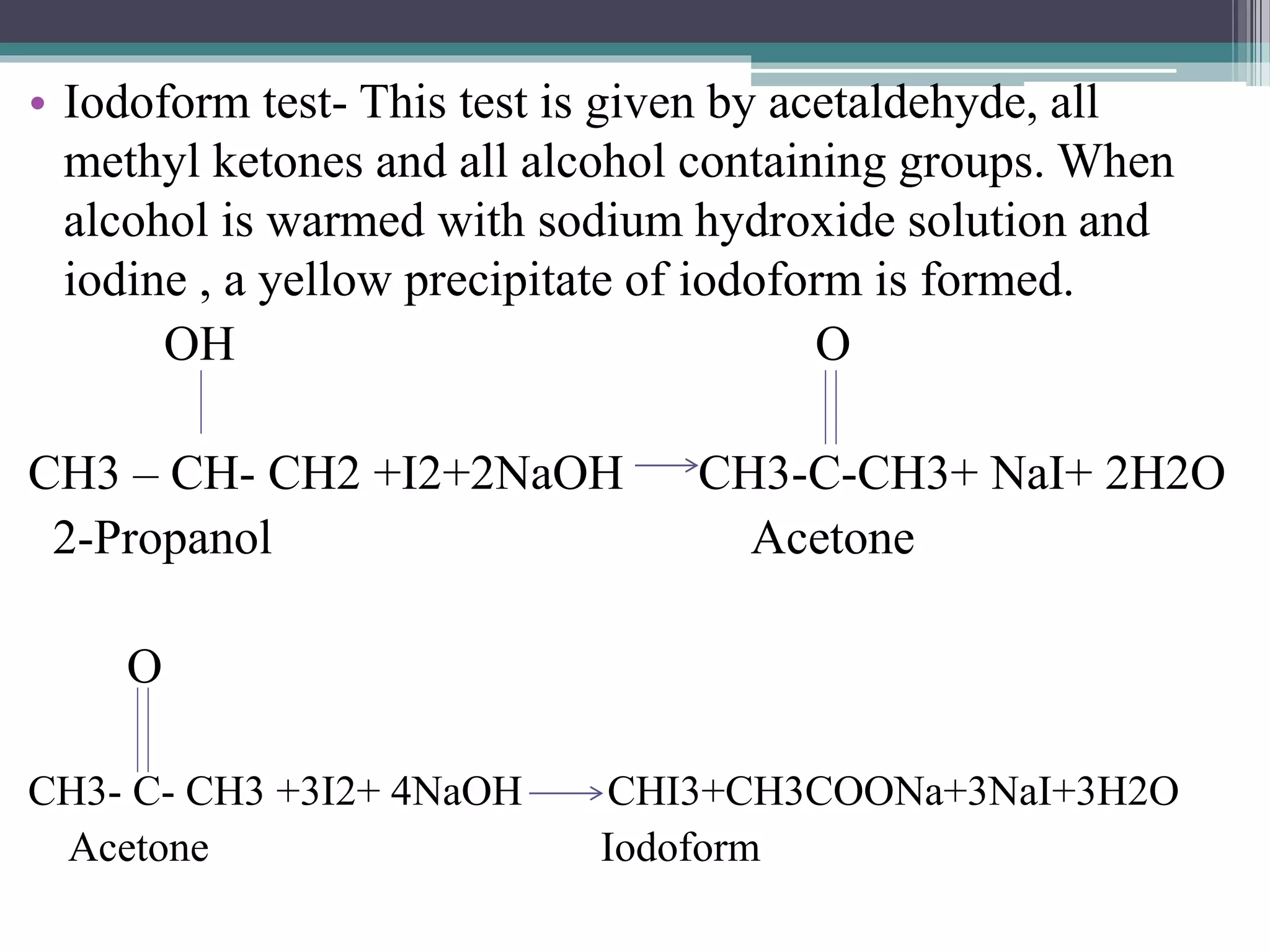
![Distinction between Primary, Secondary and Tertiary alcohols
1
• Lucas Test
2
• TCICA Test [ Trichloroisocyanuric acid]
3
• Victor Meyer Test](https://image.slidesharecdn.com/alcohols-200618145503/75/Alcohols-20-2048.jpg)



![• Steps involved in this reaction-
Step-1= The proton [H+] form Hydrochloric acid [HCl] will
protonate the OH- group of the alcohol. Water [H2O] attached
to the carbon is a weaker is a weaker nucleophile than Cl- (
Chloride). Thus, nucleophile Cl- replaces the H2O group
forming a carbocation as its present in excess.
Step-2= Cl- attacks the carbocations and the forms alkyl
chloride. +.H
: OH HCl : OH Slow + Cl- Cl
Alkyl Chloride](https://image.slidesharecdn.com/alcohols-200618145503/75/Alcohols-24-2048.jpg)


![TCICA[ Trichloroisocyanuric Acid] TEST
• This test is used to differentiate between primary and
secondary alcohol by their rate of oxidation.
• This test is conducted by adding unknown solution of
TCICA in acetonitrile 3 containing hydrochloric acid 4 and
than measuring the time for a precipitate to form.
• Primary alcohols react slowly and secondary alcohols react
rapidly.](https://image.slidesharecdn.com/alcohols-200618145503/75/Alcohols-27-2048.jpg)