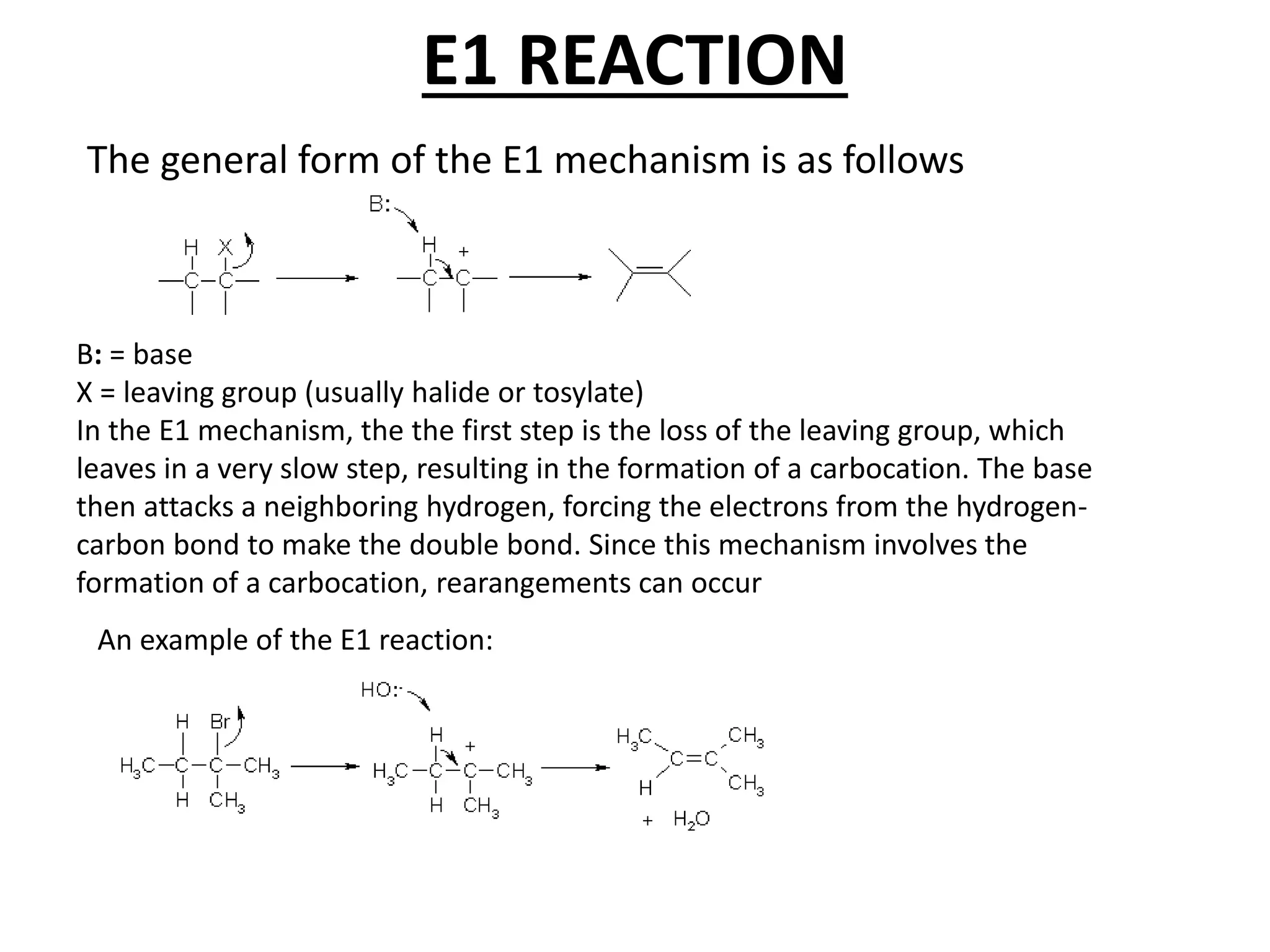
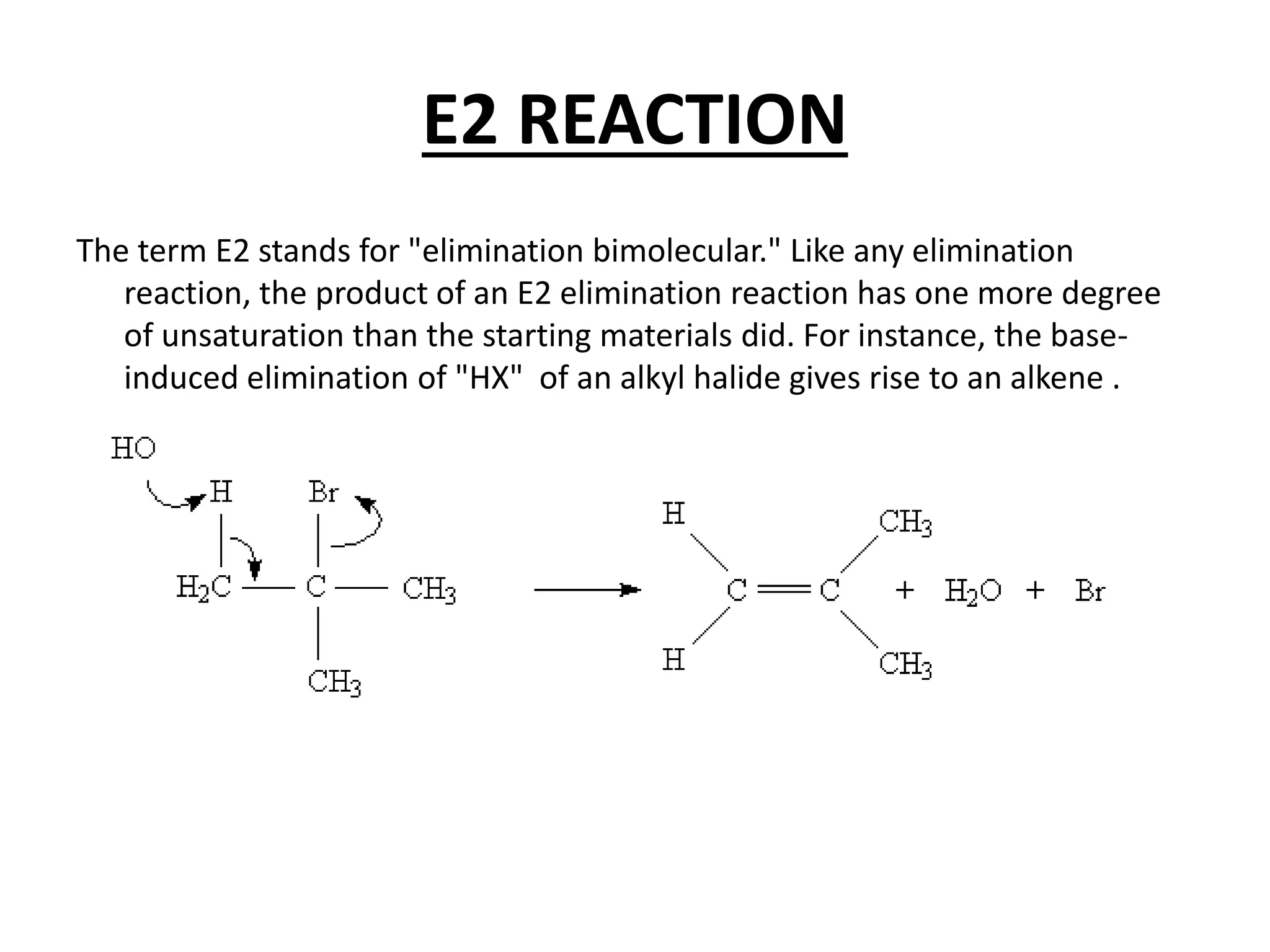
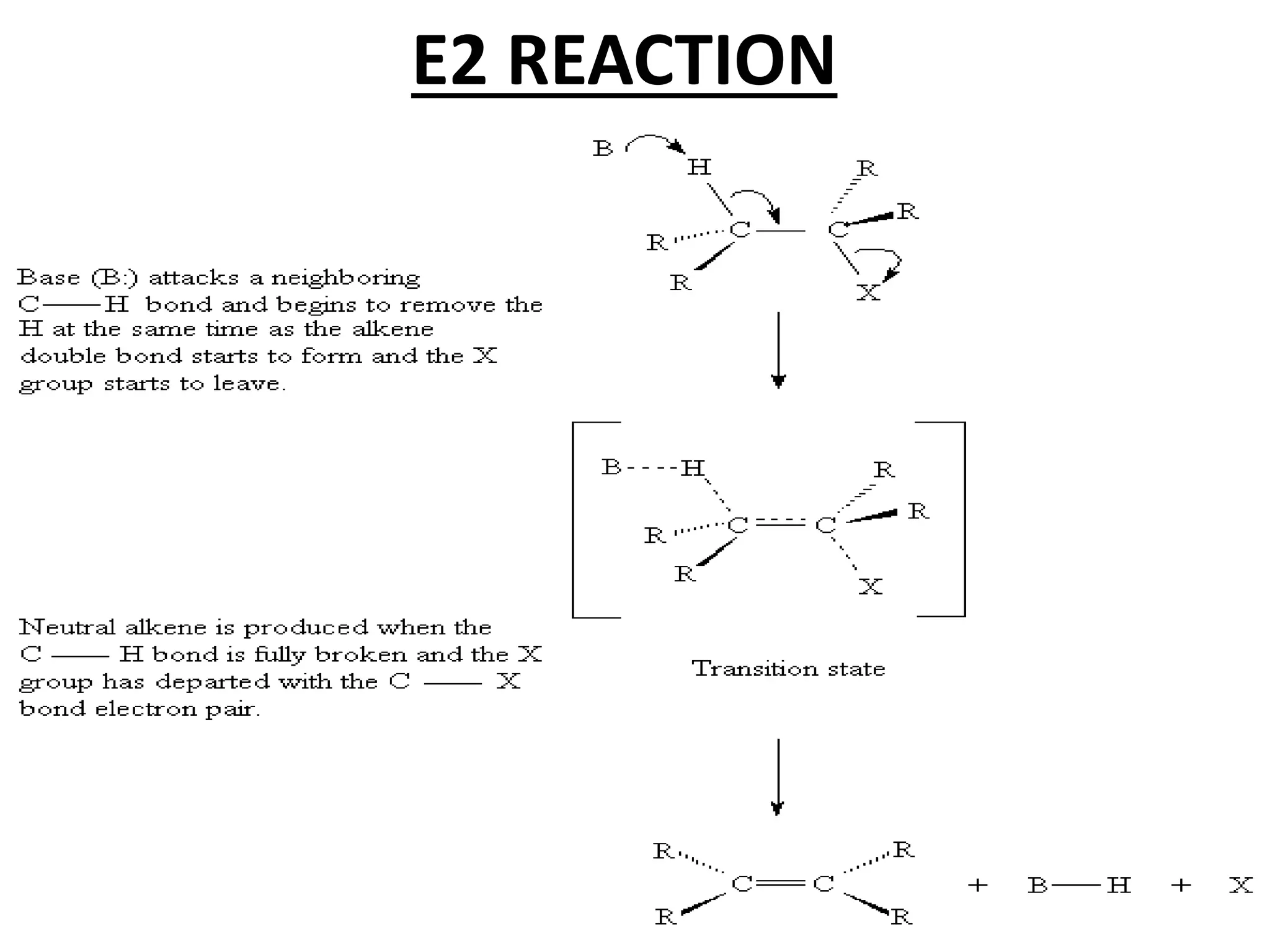
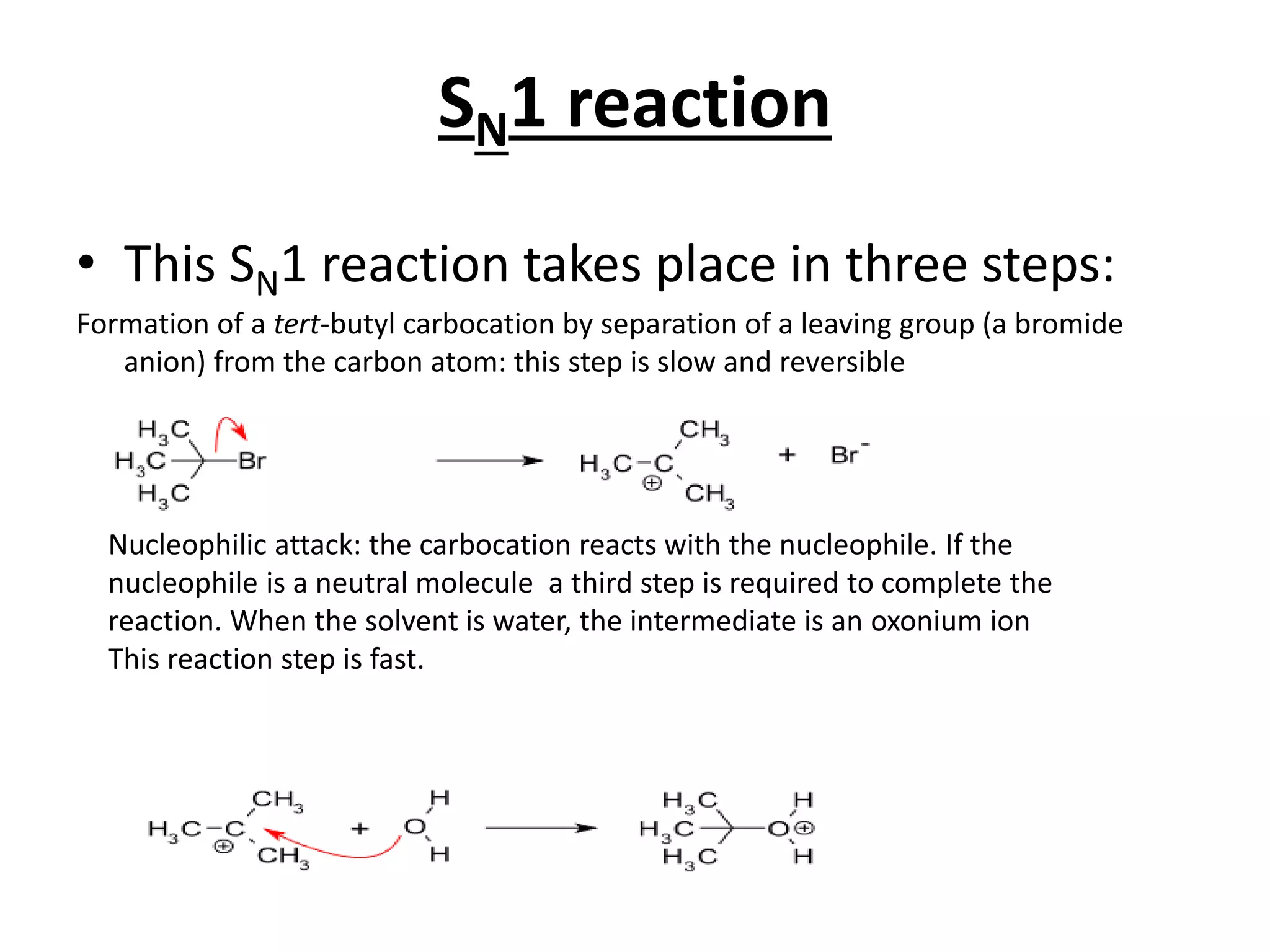
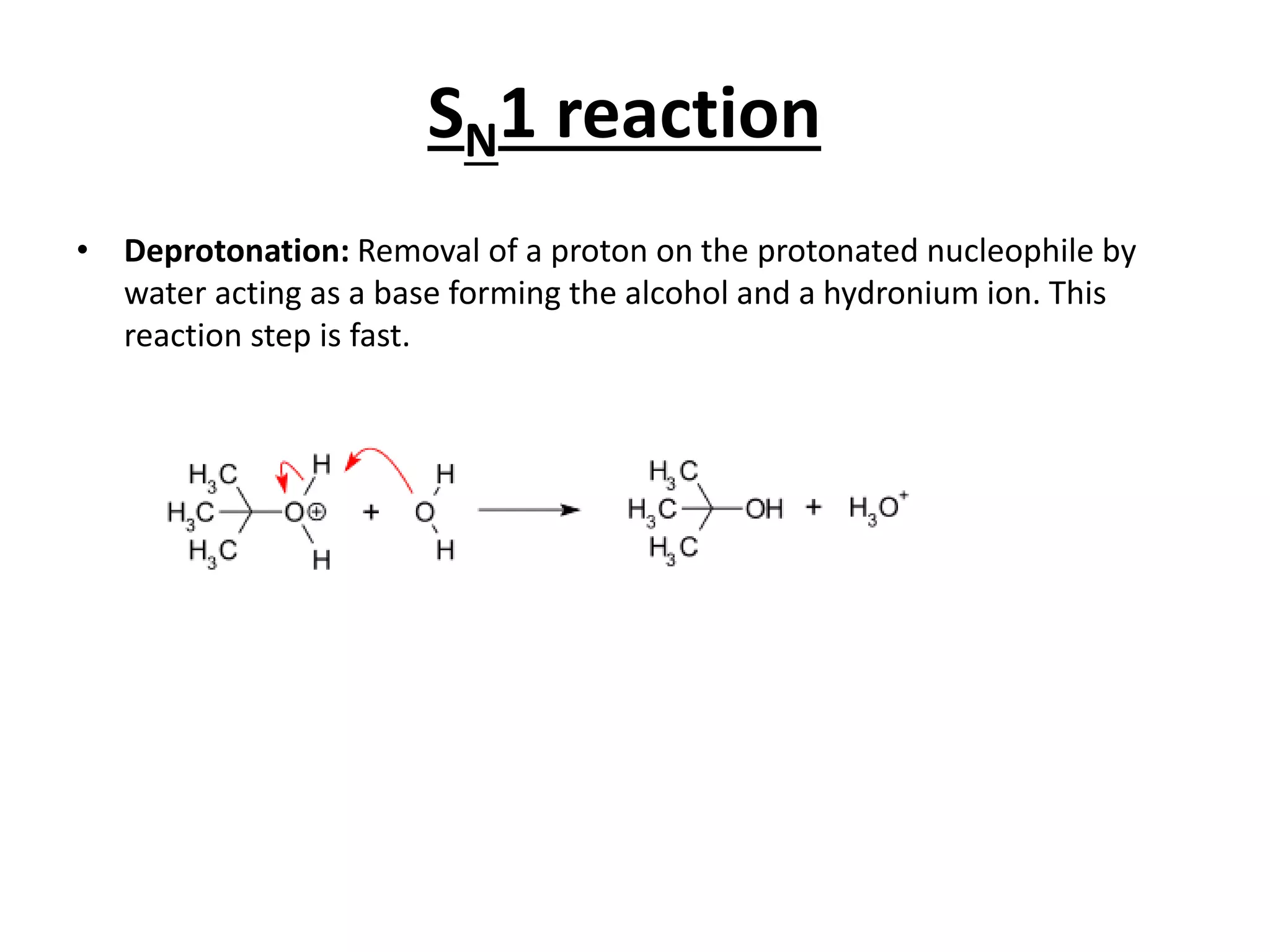
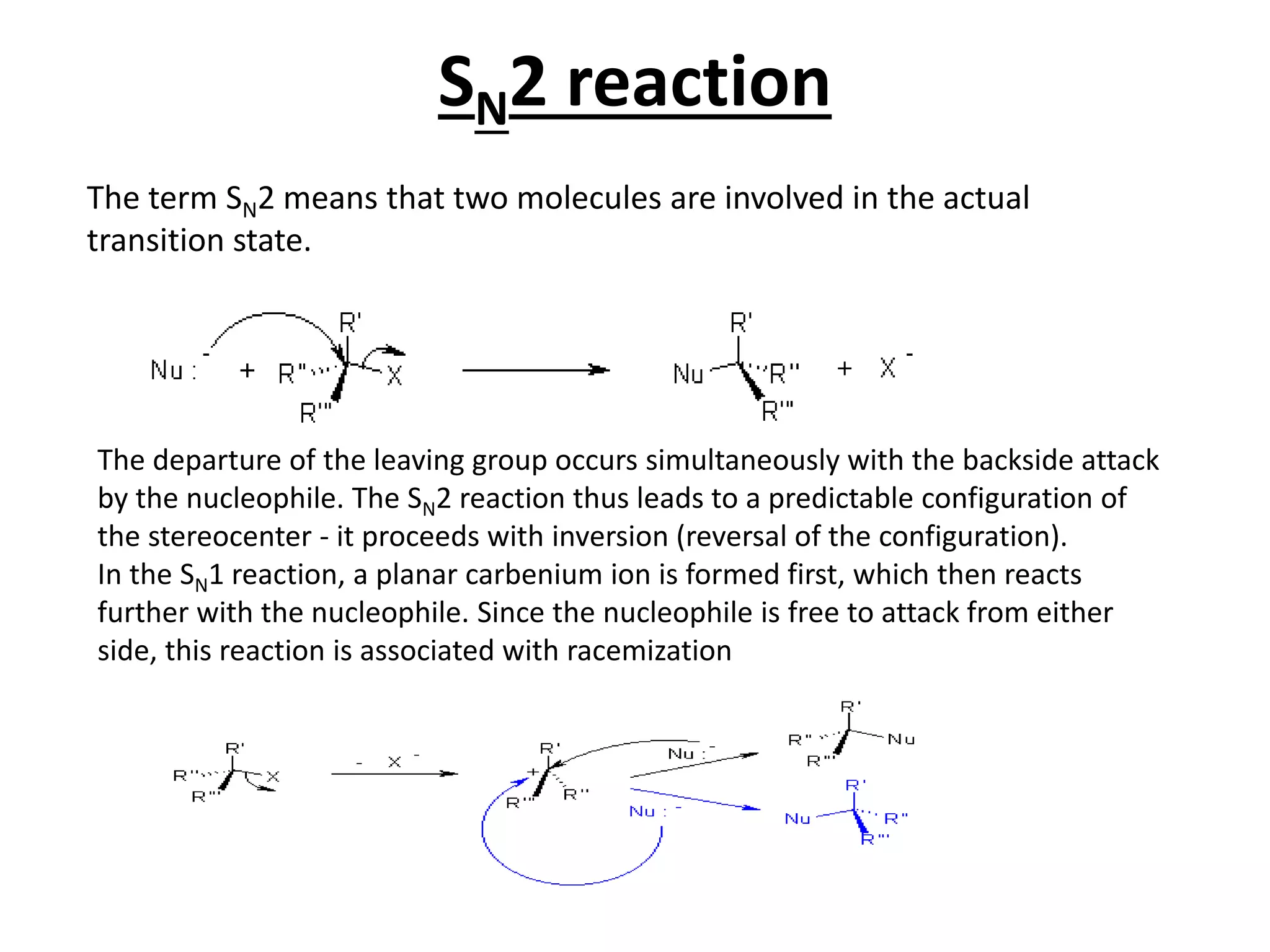
The E1 reaction involves the slow loss of a leaving group to form a carbocation intermediate. This allows rearrangements to occur. A base is not required for the rate determining step. The E2 reaction is an elimination reaction that results in a product with one more degree of unsaturation. The SN1 reaction involves the formation of a carbocation intermediate through a unimolecular rate determining step. This can allow for nucleophilic attack from either side and possible racemization. The SN2 reaction involves synchronous breaking of one bond and formation of another in one step, leading to inversion of configuration.