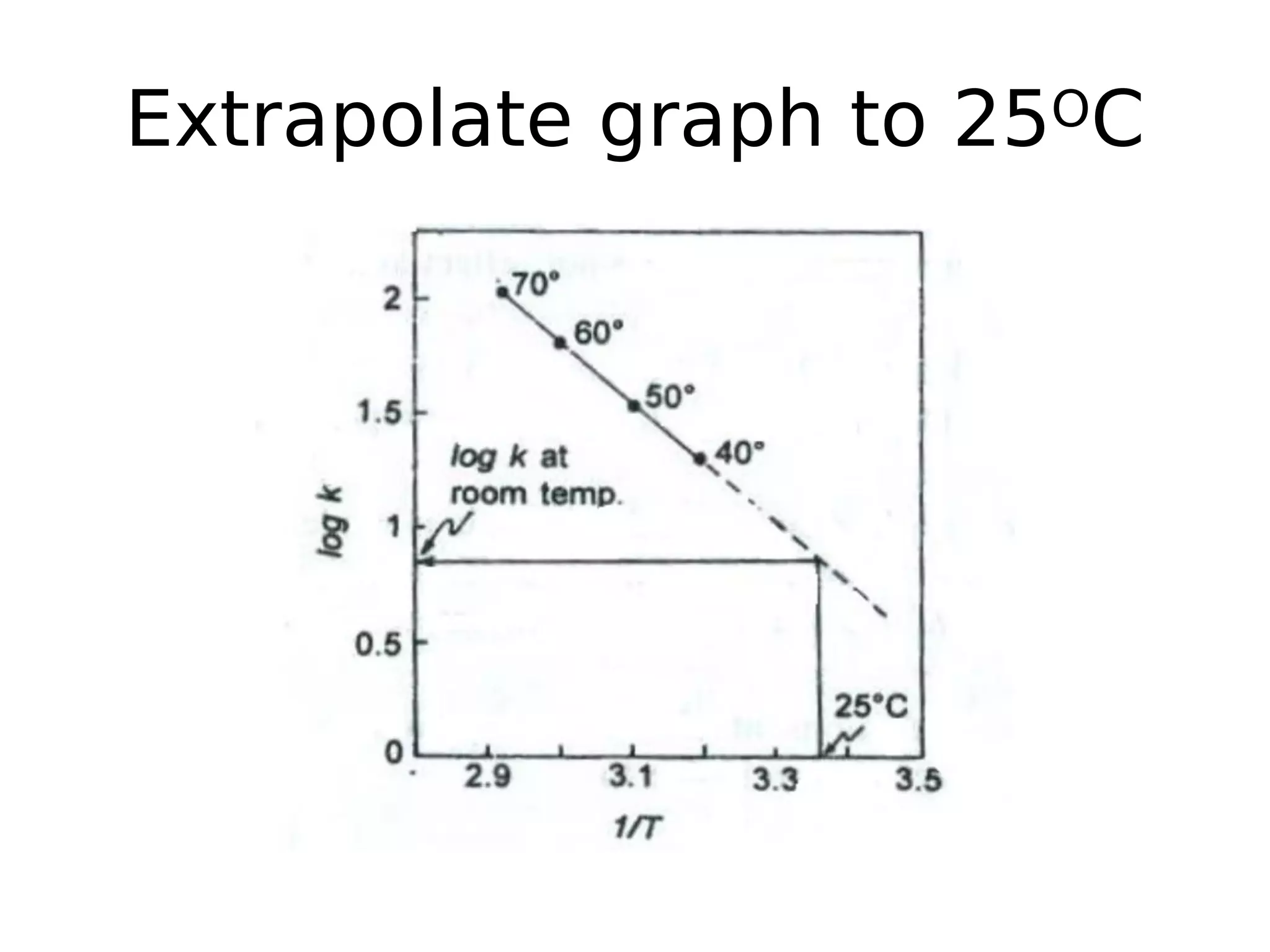
This document discusses accelerated stability testing and ICH guidelines. It provides definitions of stability testing and describes how exposing products to elevated temperatures can accelerate degradation reactions to predict long-term shelf life. Key points covered include common degradation reactions, advantages of accelerated testing, ICH guidance on test conditions, use of the Arrhenius equation to determine activation energy and calculate degradation rates at different temperatures, and equations for determining shelf life based on reaction order. Accelerated stability testing allows shelf life to be predicted in months rather than conducting long-term studies at room temperature.