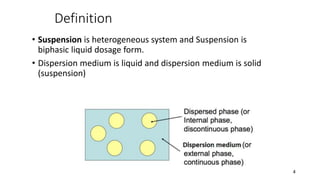
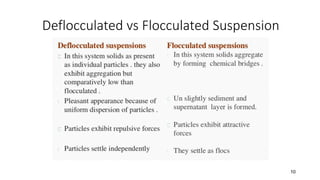
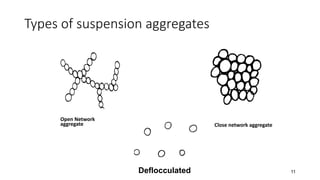
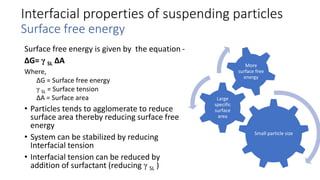
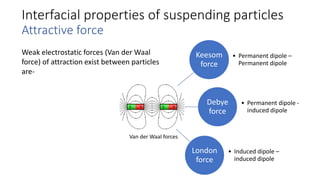
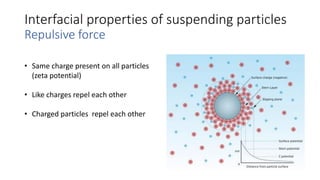
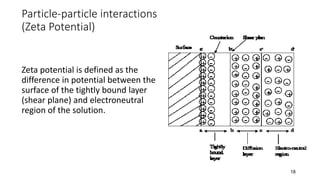
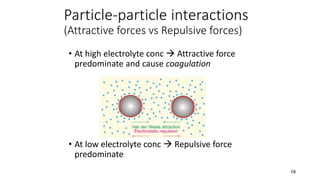
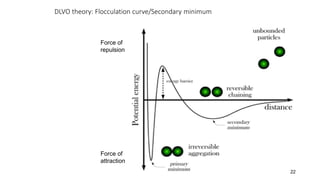
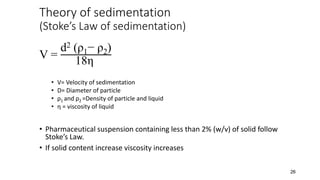
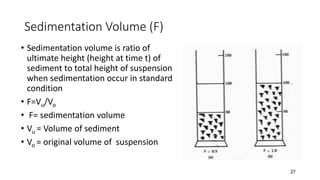
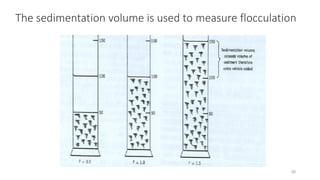
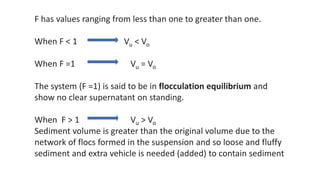
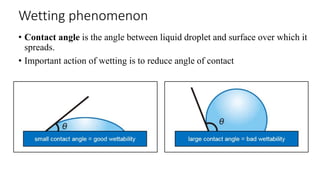
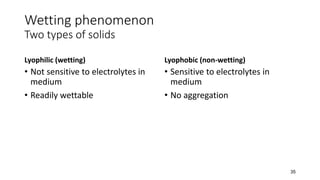
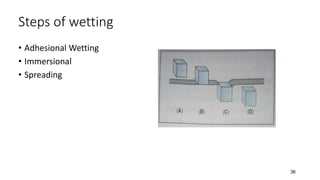
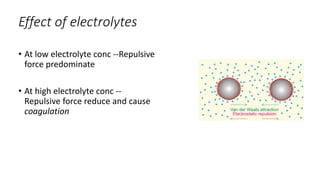
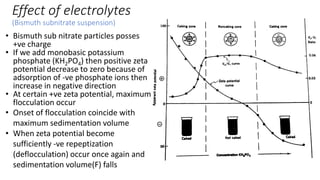
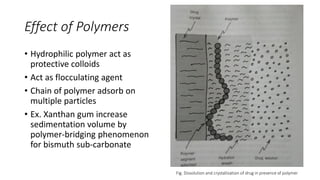
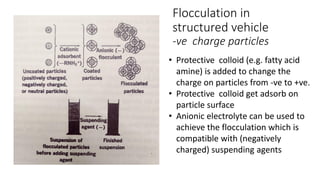
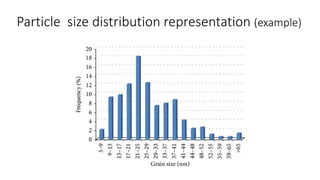
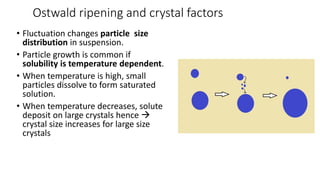
This document presents an in-depth overview of pharmaceutical suspensions, detailing definitions, desired features, formulation methods, and applications. It discusses the differences between flocculated and deflocculated suspensions, sedimentation principles, and the impact of interfacial properties on stability and performance. Furthermore, it covers techniques for improving wetting and controlled flocculation, while also addressing the effects of temperature, electrolytes, and polymers on the physical stability of suspensions.