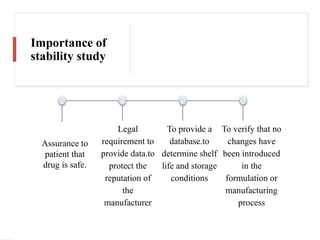
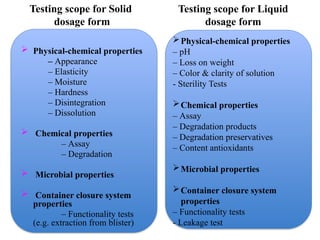
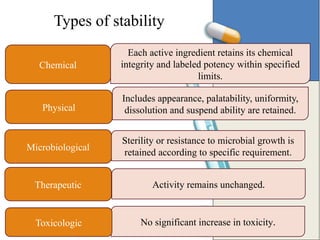
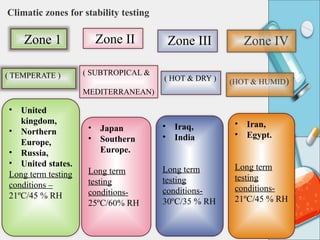
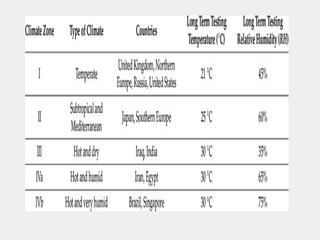
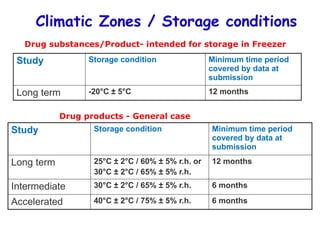
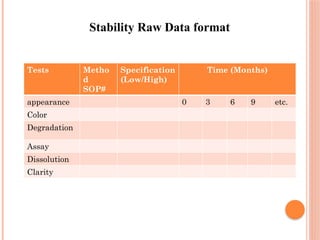
The document outlines the importance of stability testing in drug development, highlighting objectives such as establishing shelf life and ensuring patient safety. It details various testing methods, types of stability, and stages of stability studies, influenced by climatic zones. Finally, it emphasizes pharmacists' responsibilities in managing product stability and adhering to storage conditions to guarantee quality.