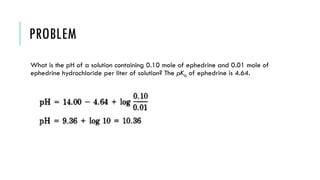
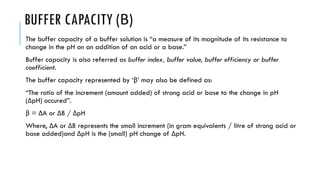
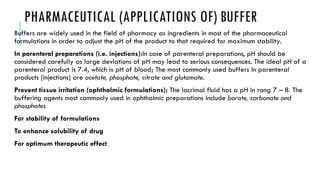
Buffer solutions resist changes in pH upon the addition of small amounts of acid or base through buffer action. A buffer is a combination of a weak acid and its conjugate base. The pH of a buffer solution depends on the ratio of the concentration of the salt to the acid based on the Henderson-Hasselbalch equation. Factors like the addition of neutral salts, dilution, and temperature can impact the pH of a buffer solution. Buffers have various applications in pharmaceutical formulations to adjust pH for stability and therapeutic effects.


![BUFFER EQUATION (HENDERSON-HESSELBALCH EQUATION)
When salt and weak acid have common ion
E.g. Sodium acetate + Acetic acid
For acid
pH = pKa + Log [Salt]/[Acid]
For base
pH = pKw - pKb + Log [Salt]/[Acid]](https://image.slidesharecdn.com/buffersb-191206070749/85/Buffer-and-Buffer-capacity-3-320.jpg)