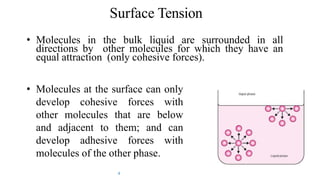
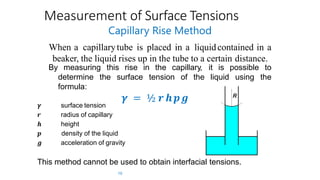
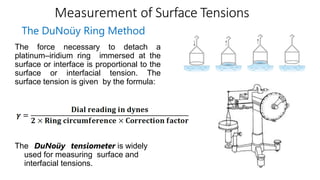
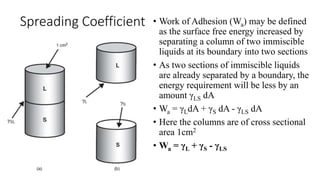
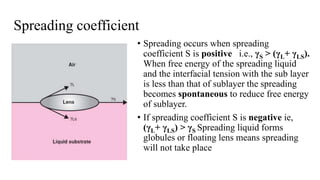
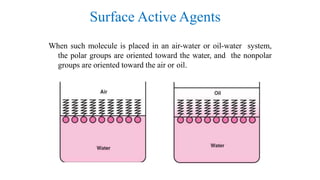
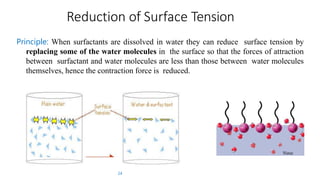
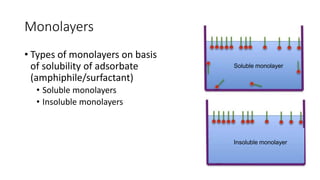
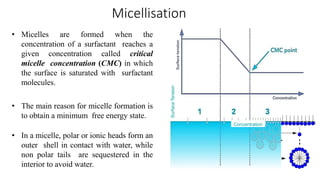
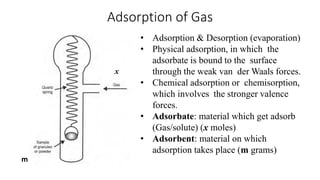
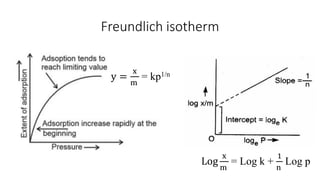
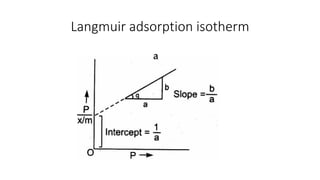
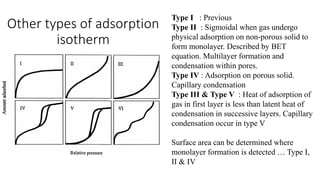
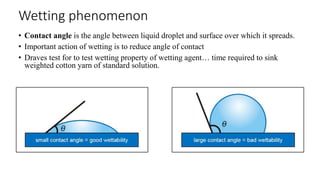
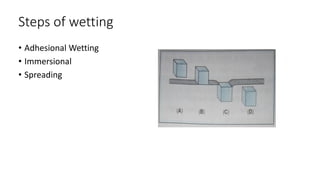
The document discusses interfacial phenomena, including surface tension, interfacial tension, and surface free energy, describing their measurement methods and implications in liquid interaction. It explains concepts such as spreading coefficients, surfactants, and adsorption processes, detailing how they influence surface activity and stability in various applications. Additionally, it covers the micellization process and the significance of contact angles in wetting behavior, providing insight into practical uses in fields like pharmacy and chemistry.