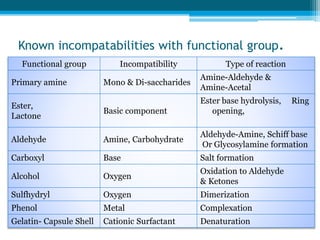
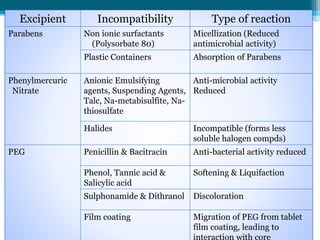
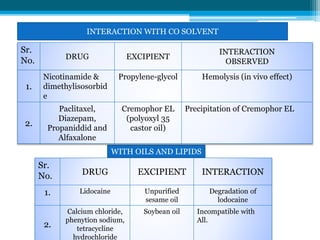
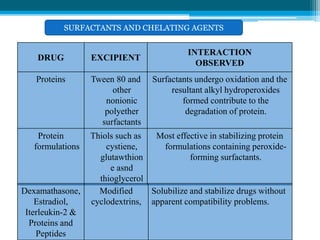
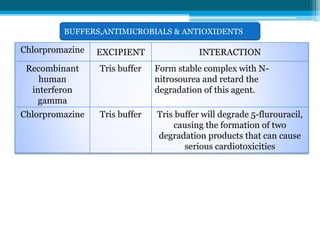
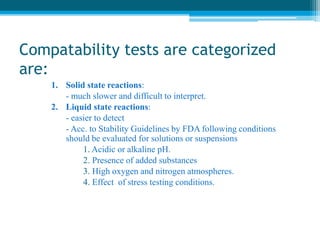
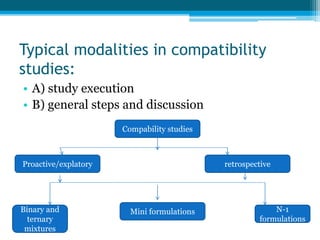
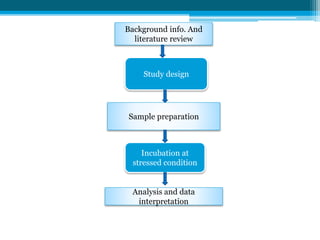
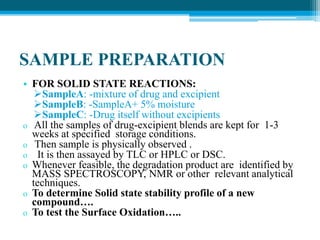
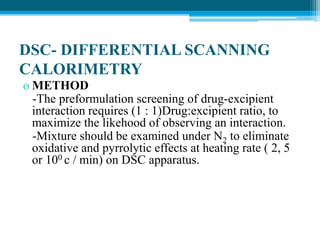
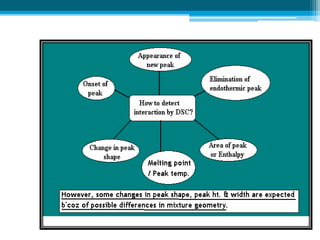
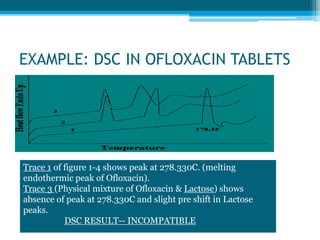
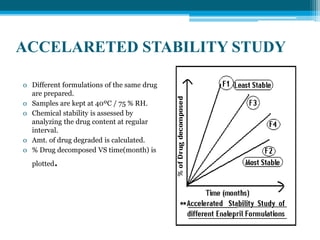
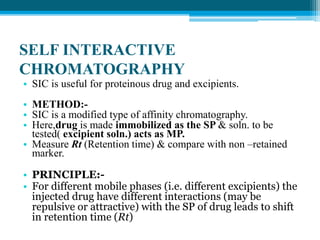
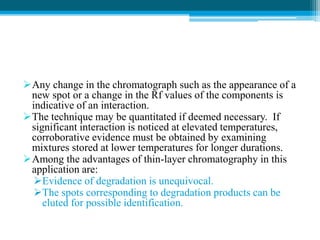
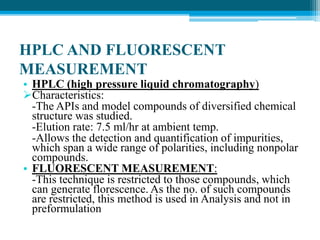
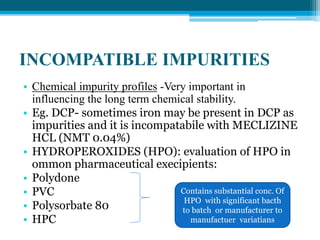
The document discusses drug excipient compatibility studies, emphasizing their importance in pre-formulation testing to ensure the stability and effectiveness of proposed dosage forms. It outlines the goals of these studies, the mechanisms of drug-excipient interactions, and various methods for assessing compatibility, such as thermal analysis and chromatography. The document also details specific excipient interactions and their effects on drug formulations, highlighting the need for comprehensive preformulation profiles to guide formulation development.