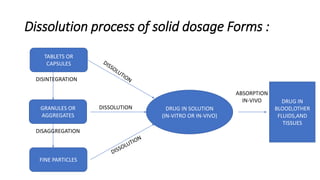
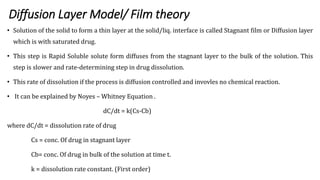
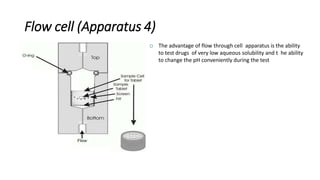
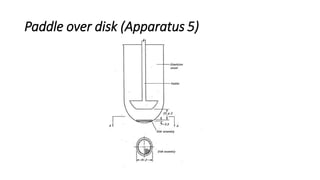
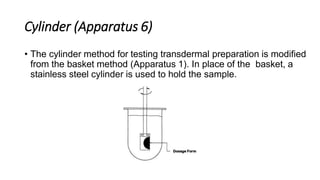
This document discusses in-vitro dissolution testing and drug release. It defines dissolution as the process where a solid substance is solubilized in a liquid solvent. Dissolution is the rate determining step for drug absorption if the drug is highly soluble. The document then covers various theories of dissolution, types of in-vitro dissolution testing models and apparatuses, and factors that can affect drug dissolution and release such as drug properties, formulation components, and test conditions.