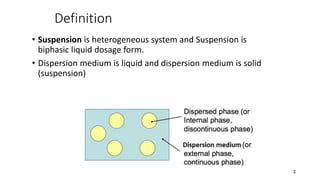
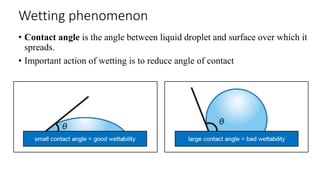
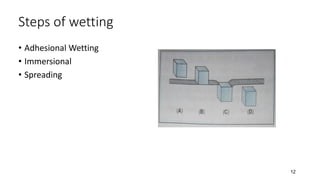
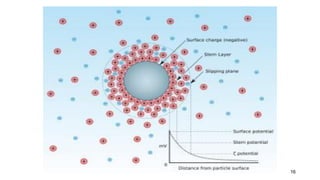
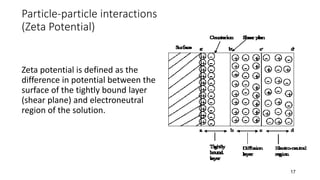
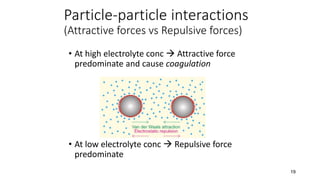
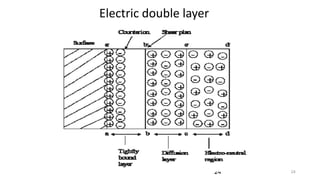
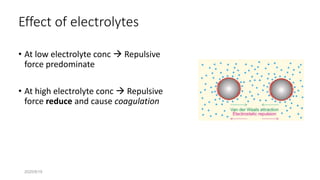
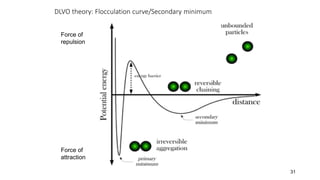
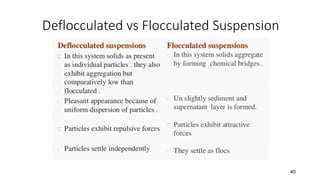
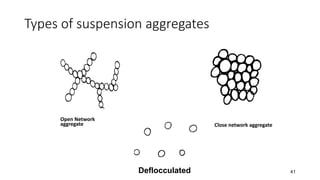
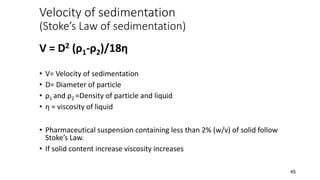
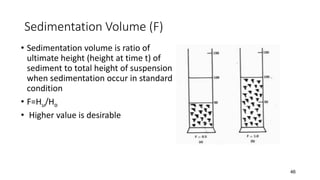
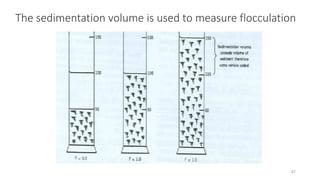
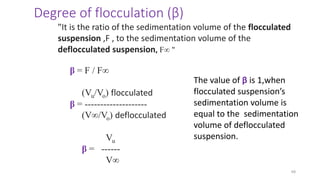
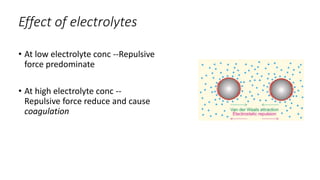
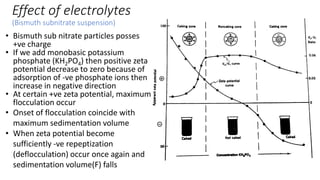
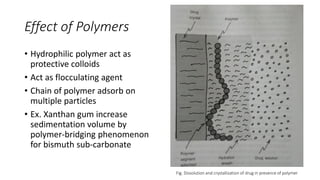
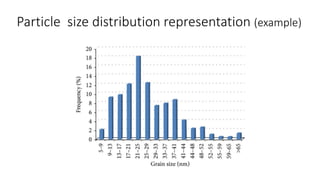
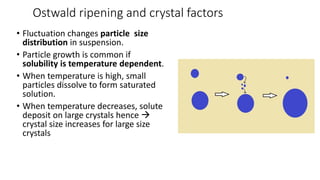
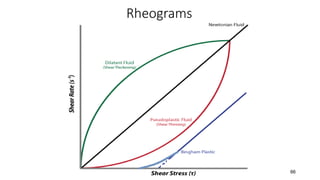
The document discusses biphasic systems, focusing on suspensions in pharmaceuticals, detailing their properties, advantages, and disadvantages. It explains the significance of particle interactions, wetting phenomena, sedimentation processes, and the principles of flocculation and deflocculation. Additionally, it covers theories like DLVO, the effect of electrolytes, and strategies for achieving stable and effective suspensions in drug formulations.