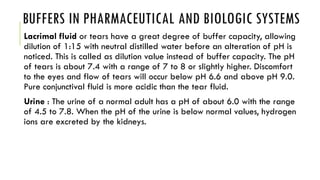
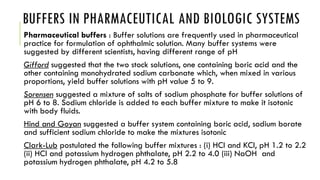
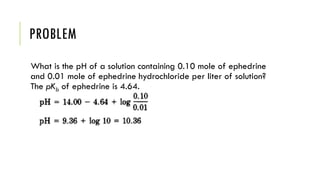
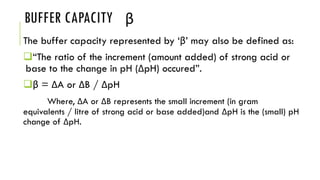
Buffer solutions resist changes in pH upon addition of small amounts of acid or base. They are made up of a weak acid and its conjugate base. Buffers have important applications in pharmaceutical manufacturing and drug formulations. The pH of buffer solutions and how much they resist pH changes can be calculated using the Henderson-Hasselbalch equation. Factors like temperature, dilution, and addition of salts can impact buffer solutions. Biological fluids also use buffer systems, like bicarbonate buffers in blood and phosphate buffers in tears, to maintain optimal pH ranges.




![BUFFER EQUATION (HENDERSON-HESSELBALCH EQUATION)
E.g. Sodium acetate + Acetic acid
When salt (Sodium acetate) and weak acid (Acetic acid) have common
ion (acetate), it can form buffer solution
Equation for w. acid
pH = pKa + Log [Salt]/[Acid]
Equation for w. base
pH = pKw - pKb + Log [Base]/[Salt]](https://image.slidesharecdn.com/buffersb-201106132834/85/Buffer-Applications-and-capacity-SB-6-320.jpg)

![BUFFERS IN PHARMACEUTICAL AND BIOLOGIC SYSTEMS
Blood is maintained at a pH of about 7.4 by the so-called primary buffers
in the plasma and the secondary buffers in the erythrocytes
The plasma contains carbonic acid/bicarbonate as buffers. Plasma proteins,
which behave as acids in blood, can combine with bases and so act as
buffers.
In the erythrocytes, the two buffer systems consist of
hemoglobin/oxyhemoglobin
The dissociation exponent pK1= 6.1 for ionization of carbonic acid in the
plasma at body temperature
The buffer equation for the carbonic acid/bicarbonate buffer of the blood
is
pH = 6.1 + log
[HCO3−]
[H2CO3]](https://image.slidesharecdn.com/buffersb-201106132834/85/Buffer-Applications-and-capacity-SB-8-320.jpg)