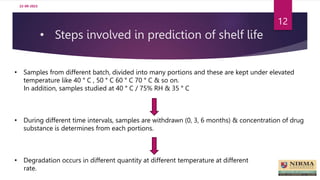
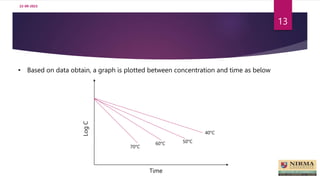
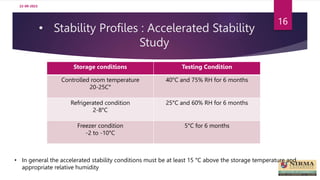
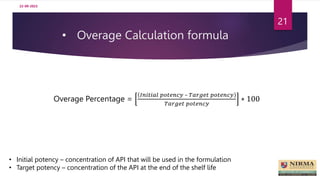
Accelerated stability studies are performed to determine the shelf life of a drug product by exposing it to exaggerated stress conditions as per ICH guidelines. The studies follow the Arrhenius equation to correlate degradation rates at elevated temperatures to predicted rates at normal storage conditions. Samples are withdrawn from batches stored at different temperatures and humidity over time for analysis. Degradation kinetics are used to calculate shelf life and determine the need for overages to maintain drug content throughout shelf life. While rapid, accelerated studies have limitations when degradation mechanisms differ from those at normal storage conditions.









![22-09-2023
15
• Converting into Log10
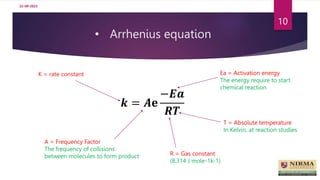
Log k = -Ea/2.303 RT + Log A
Y = m x + c
• A graph is plotted between Log k and its corresponding
reciprocal of temp (1/T).
• The plot is extrapolated to room temperature, 25 ° C to determine k value.
• This k value is substituted into shelf life equation [t90 = 0.105/k ] to determine
shelf life of product.](https://image.slidesharecdn.com/acceleratedstabilitystudies-230924114149-c2e8147b/85/Accelerated-Stability-Studies-pptx-15-320.jpg)




![ REFERENCES
22-09-2023
22
1. ICH Quality Guidelines - Q1A(R2) – referred on 16-09-2023
2. Drug Stability : Principles and Practices, J.T. Carstensen, C.T. Rhodes. New York Marcel Dekker Publication [3rd
edition]
3. “Pharmaceutics the science of dosage form design”, “Kinetics and stability testing, Aulton M.E London
Churchill Livingstone Publication [2nd edition]
4. Pharmaceutical dosage forms tablets, Herbert A. Lieberman, Leon Lachman, Joseph B. Schwartz. New
York Marcel Dekker Publication - Volume 3 [2nd edition]
5. Drug Stability : ICH versus Accelerated Predictive Stability Studies by Olga Gonzalez-Gonzalez
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9693625/](https://image.slidesharecdn.com/acceleratedstabilitystudies-230924114149-c2e8147b/85/Accelerated-Stability-Studies-pptx-22-320.jpg)
