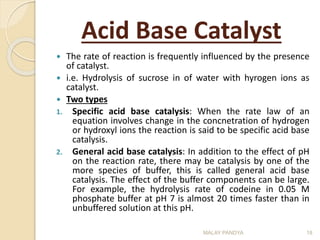
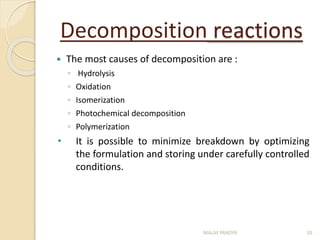
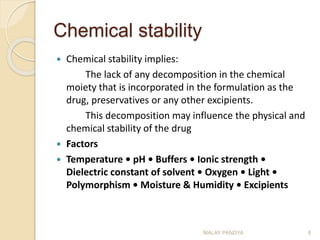
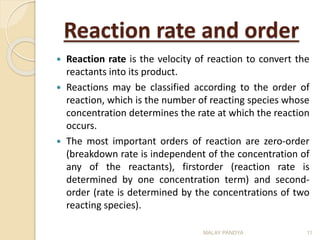
The document discusses the stability of pharmaceuticals and the importance of pharmaceutical formulation in converting drugs into effective medicines. It highlights factors affecting stability, such as temperature, humidity, and light, and outlines potential consequences of instability, including loss of active ingredients and changes in bioavailability. Additionally, it covers different types of stability studies, including physical, chemical, and microbiological stability, as well as chemical reaction rates and the effects of catalysts on drug decomposition.




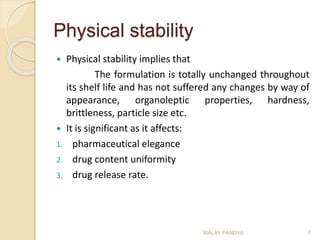


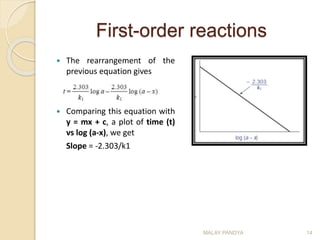
![First-order reactions
As the name suggests the rate depends on the
concentration of one reactant.
The rate equation is
Integrating this equation with respect to time from t=0 to t=t
Where a is the amount of drug degraded at time t, x is the
initial amount of drug, k1 is the rate of reaction.
The rate of decomposition of a drug A is the change of
concentration of A over a time interval, t, i.e., –d[A]/dt (note
that this is negative because the drug concentration is
decreasing). However, it is more usual to express the rate as
dx/dt, where x is amount of drug which has reacted in time t.
MALAY PANDYA 13](https://image.slidesharecdn.com/stability-181021062456/85/Stability-of-Pharmaceuticals-13-320.jpg)
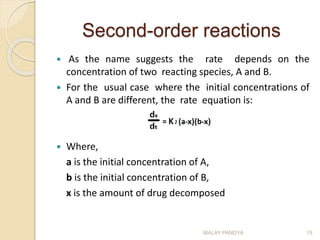
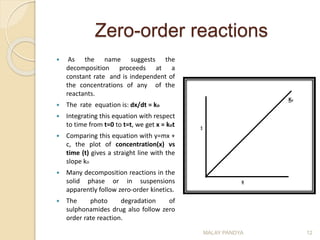
![Second-order reactions
Integrating this equation with
respect to time from t=0 to t=t,
Rearrangement of the above
equation gives
Comparing this equation with
y = mx + c, a plot of time (t) vs
log [(a-x)/(b-x)], would yield a
straight line with
slope = 2.303/k2(a – b).
MALAY PANDYA 16](https://image.slidesharecdn.com/stability-181021062456/85/Stability-of-Pharmaceuticals-16-320.jpg)