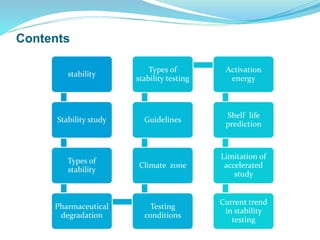
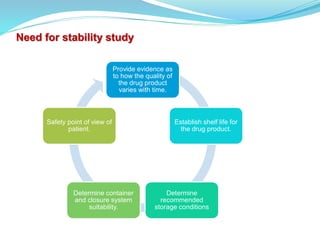
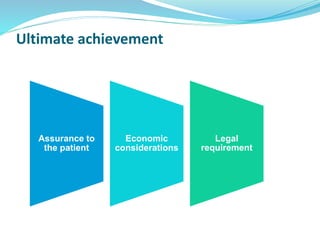
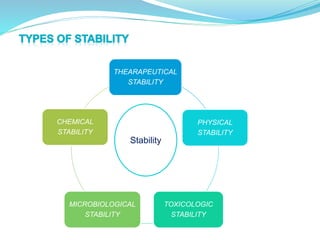
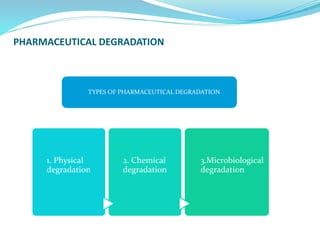
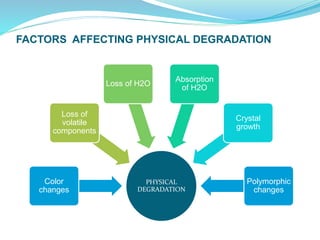
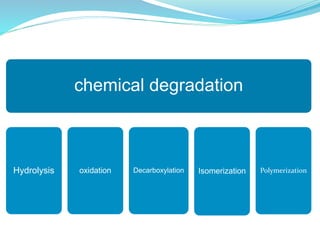
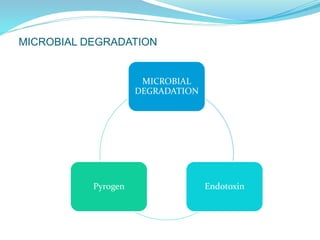
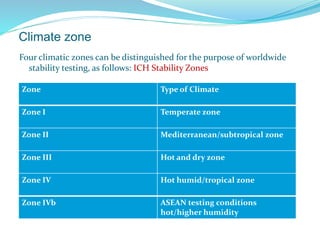
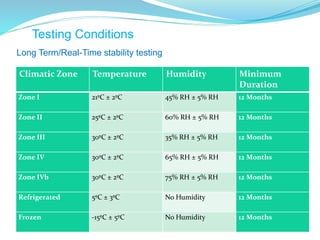
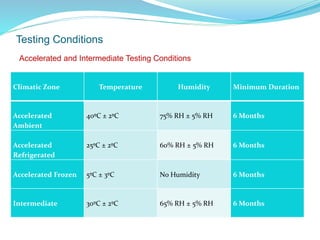
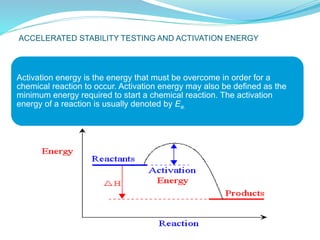
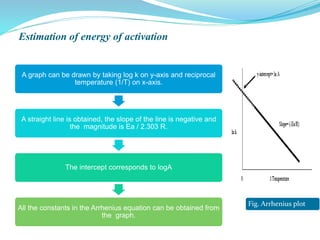
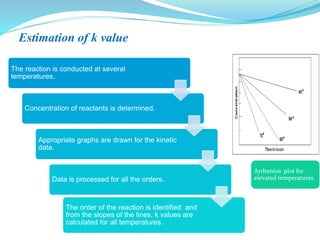
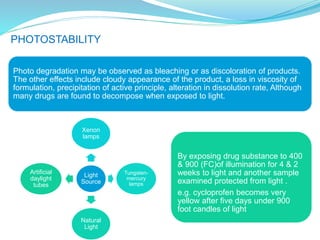
The presentation covers the stability study of pharmaceutical products, detailing types of stability, degradation factors, and testing conditions across various climate zones. It emphasizes the need for stability studies to establish shelf life, ensure patient safety, and comply with legal requirements. Current trends in stability testing include defining global testing conditions and increasing the duration of accelerated studies to optimize resources and avoid repetitive testing.