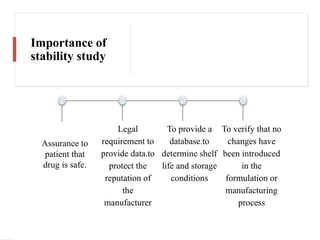
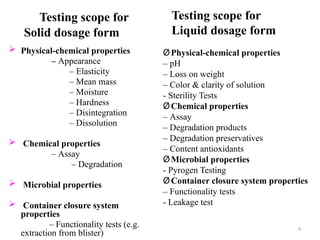
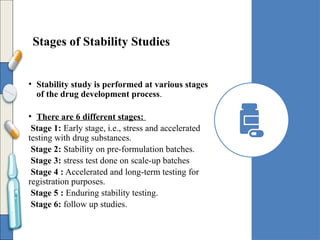
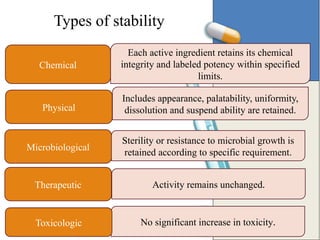
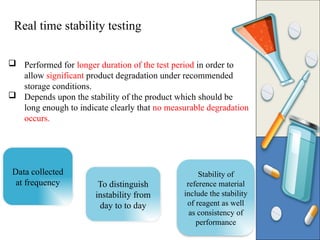
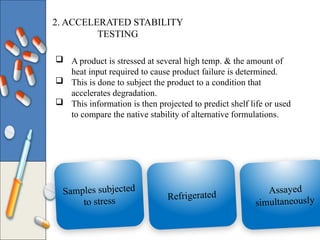
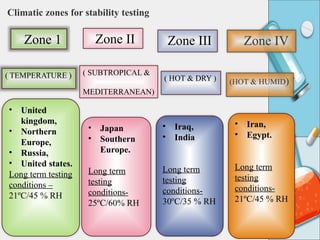
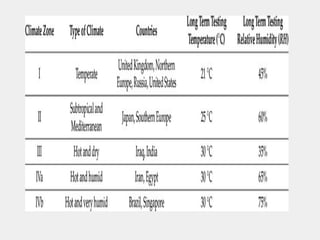
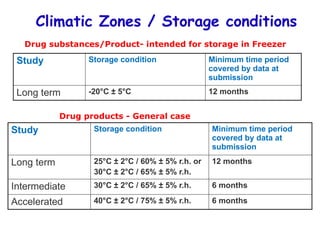
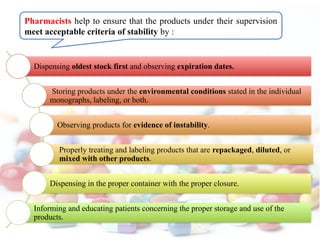
The document discusses the importance and methodology of stability studies for pharmaceutical products, focusing on determining shelf life, evaluating product quality, and ensuring patient safety. It outlines various types of stability, factors affecting stability, testing methods, and the responsibilities of pharmacists in managing product stability. The document emphasizes the need for controlled storage conditions and regulatory compliance to maintain the safety and efficacy of drugs throughout their shelf life.