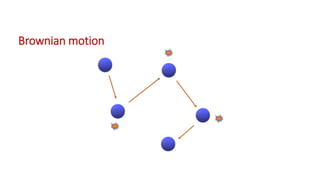
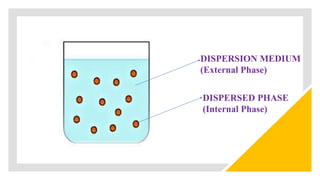
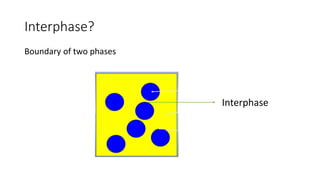
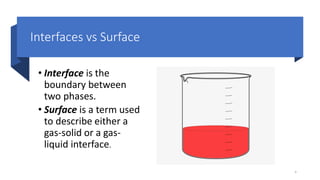
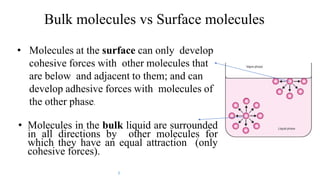
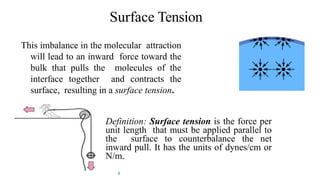
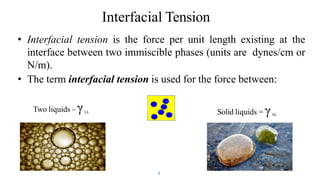
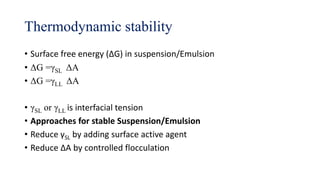
This document discusses biphasic systems, which are systems with two immiscible phases where one substance is dispersed throughout another. It defines key terms like dispersed phase, continuous phase, interface, surface tension, and interfacial tension. Stability is important for biphasic systems like suspensions and emulsions. Systems can be stabilized thermodynamically by reducing interfacial tension or surface area, or kinetically through Brownian motion and controlling sedimentation rate. Finally, it outlines desired features for dispersed systems like ease of redispersion, stability, and pleasing sensory properties.




![Gibbs free energy G,
•G = [Difference in bond energies or attractive
energies between products and reactants, H]-
[Change in probability during the process, T S]
•G=H-TS
• Where,
• H= Enthalpy
• S= Entropy
• T= Temperature](https://image.slidesharecdn.com/introductionbiphasicsystemsuspensionemulsion-200918180926/85/Introduction-biphasic-system-suspension-emulsion-12-320.jpg)


![Where,
• vsed. = sedimentation velocity in cm / sec
• d = diameter of particle
• ρ s= density of disperse phase
• ρ o= density of disperse media
• g = acceleration due to gravity
• η= viscosity of disperse medium in
poise
Stokes Equation
16
All the factors which reduce sedimentation velocity[vsed], provide kinetic
stability](https://image.slidesharecdn.com/introductionbiphasicsystemsuspensionemulsion-200918180926/85/Introduction-biphasic-system-suspension-emulsion-16-320.jpg)