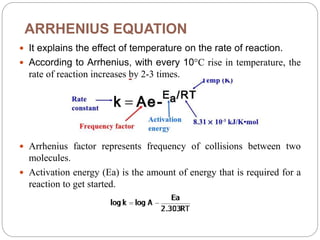
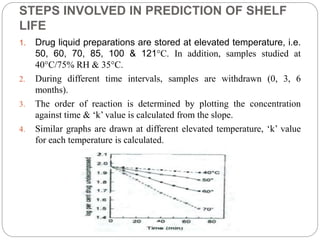
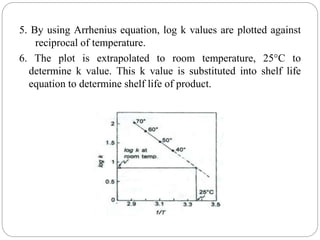
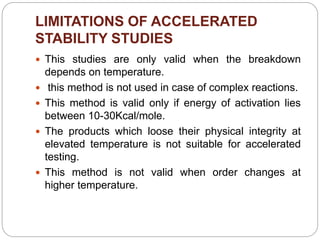
Accelerated stability studies assess product shelf life by simulating decomposition through elevated temperatures, humidity, and light. Utilizing the Arrhenius equation, key steps include storing drug preparations at various temperatures, calculating reaction rates, and extrapolating data to predict shelf life at room temperature. Limitations include applicability only to temperature-dependent reactions and in situations where the product maintains integrity under stress.