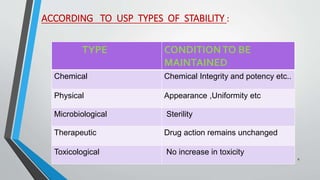
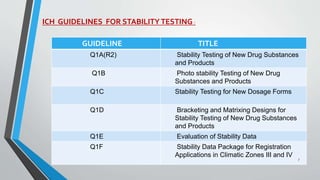
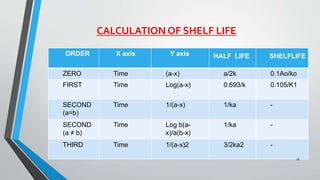
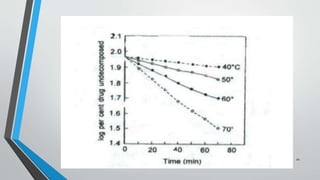
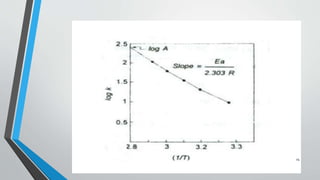
The document discusses accelerated stability studies for pharmaceutical dosage forms, emphasizing the importance of stability in maintaining the product's quality over time. It outlines methods for predicting shelf life using the Arrhenius equation, various stability tests under different conditions, and the necessity of adding overages to ensure drug potency. Additionally, it highlights guidelines and limitations of stability testing and concludes by underscoring the significance of stability knowledge for pharmaceutical formulations.