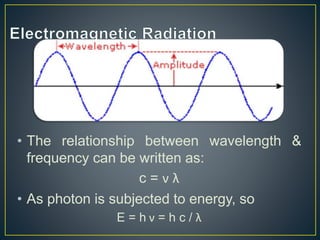
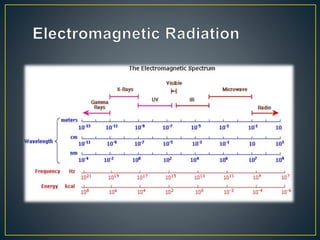
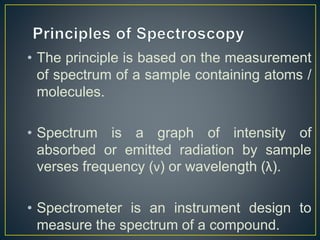
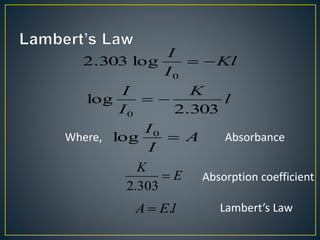
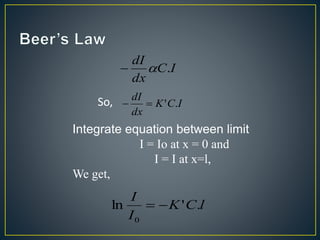
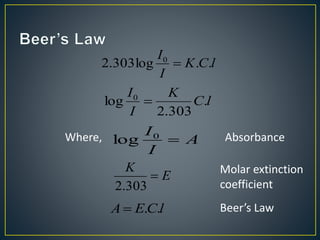
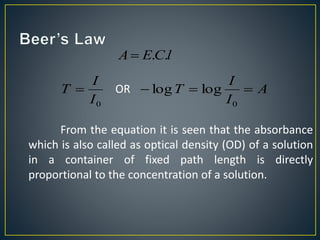
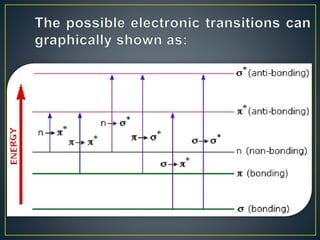
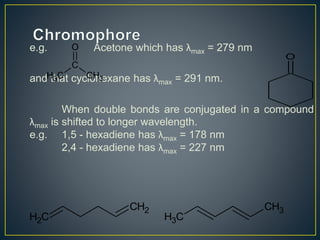
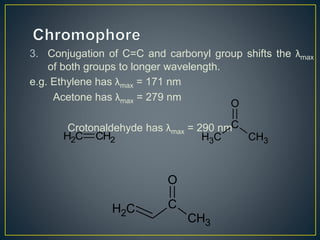
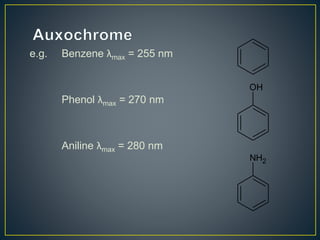
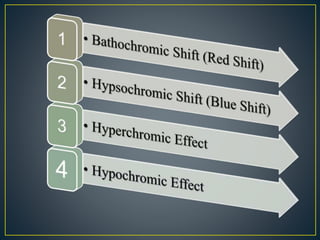
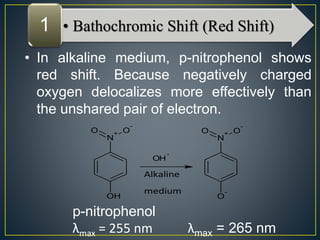
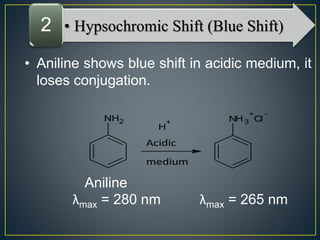
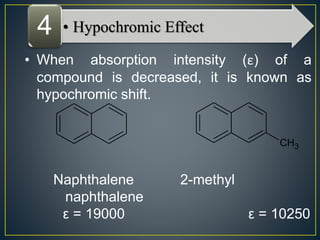
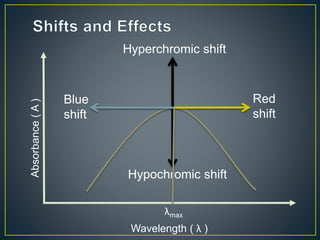
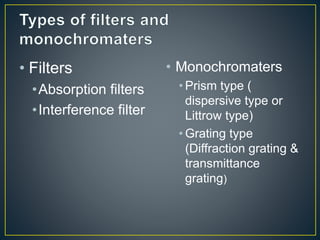
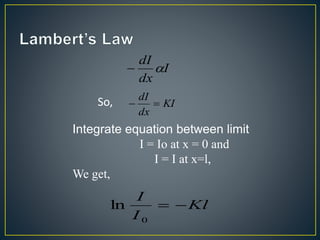This document provides an overview of spectroscopy. It discusses topics like electromagnetic radiation, photons, wavelength, frequency, the electromagnetic spectrum, absorption spectroscopy, emission spectroscopy, Lambert's law, Beer's law, chromophores, auxochromes, shifts in absorption spectra, and components of a visible spectrophotometer like sources, filters, and monochromators.