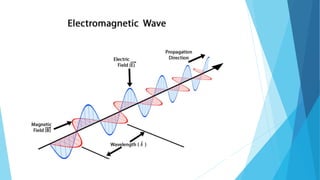
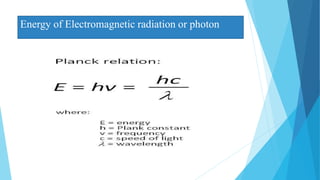
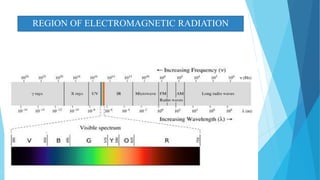
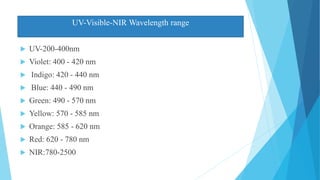
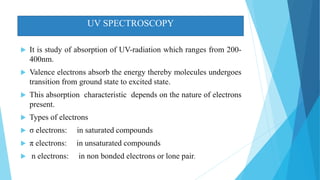
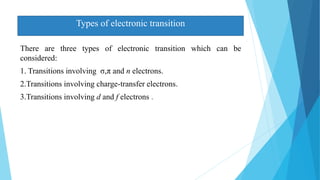
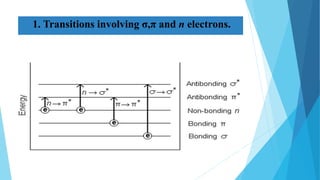
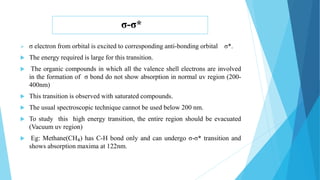
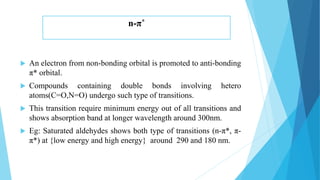
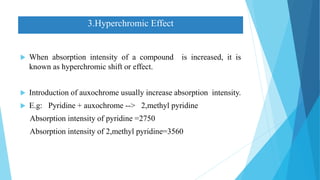
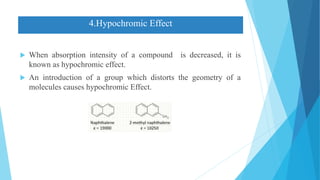
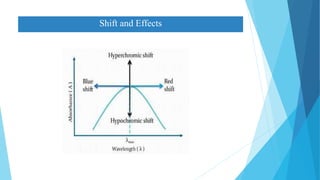
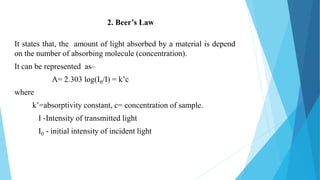
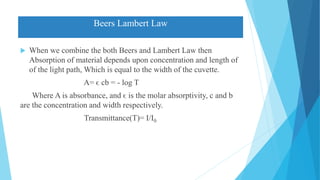
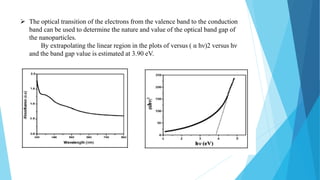
This document provides an overview of UV-Visible-NIR spectroscopy. It begins with an introduction to electromagnetic radiation and the electromagnetic spectrum. It then discusses various spectroscopy techniques including UV spectroscopy, visible spectroscopy, and NIR spectroscopy. It covers topics such as electronic transitions, terms used in UV-Vis spectroscopy like chromophores and auxochromes, Beer's law, instrumentation, and applications. Some key applications discussed include photo degradation of dyes using photocatalysis, measuring the band gap of TiO2 powder, and estimating the optical properties of nanoparticles.