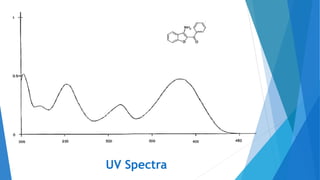
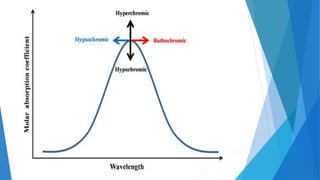
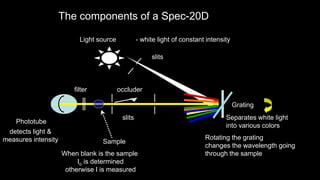
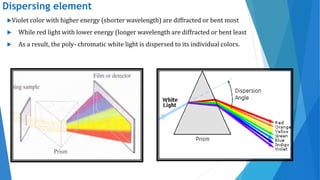
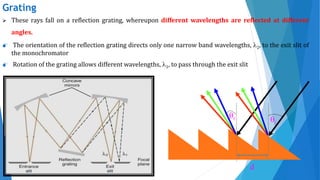
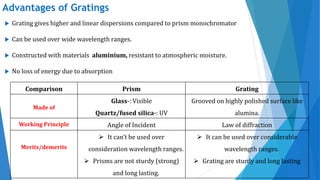
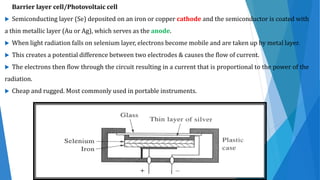
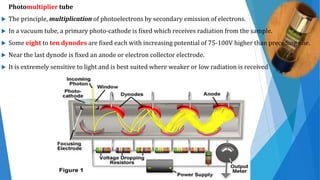
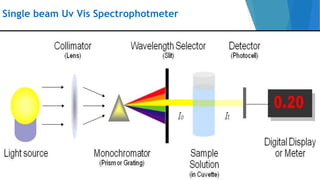
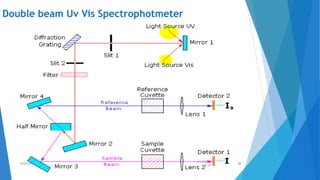
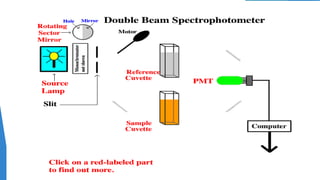
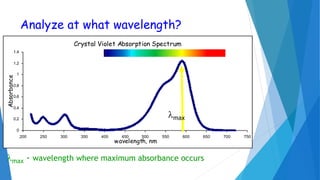
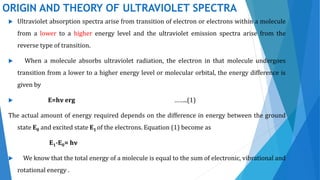
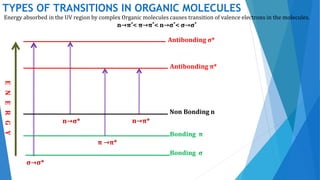
UV/Visible spectroscopy involves electronic transitions that absorb light in the ultraviolet-visible region. There are several types of transitions including n→π*, π→π*, and σ→σ* transitions. The energy and wavelength of absorbed light depends on the difference between molecular orbital energies. Chromophores and auxochromes determine absorption properties, and solvents, concentration, and temperature can affect observed spectra. UV/Vis spectrometers contain a light source, monochromator, sample holder, and detector to measure absorption of light by a sample.








![CHROMOPHORE
Chromophore is defined as any group which exhibits absorption of electromagnetic
radiations in the visible or ultraviolet region.
Important chromophore are ethylenic, carbonyls, ester, acid, nitrile group etc
Two types of chromophore are known;
a ) chromophore in which the group is having π electrons π undergo π π* transition . Example
of having such chromophore are ethylene, ester. [ Also called Dependent chromophore When more
then one chromophore is required to produce color. Eg acetone having 1 kentone group is colorless
where as diacetyl having two kentone group is yellow ]
b) Chromophore, having both π electron and {non –bonding} electrons undergo two type of
transition, i.e, π π* and n π*. Example carbonyls, nitrile, azo compound, nitro compound.
[Also called Independent chromophore: single chromophore is sufficient to import color to the
compound ]](https://image.slidesharecdn.com/uvabsorptionspectroscopy-170914111128/85/Uv-absorption-spectroscopy-11-320.jpg)