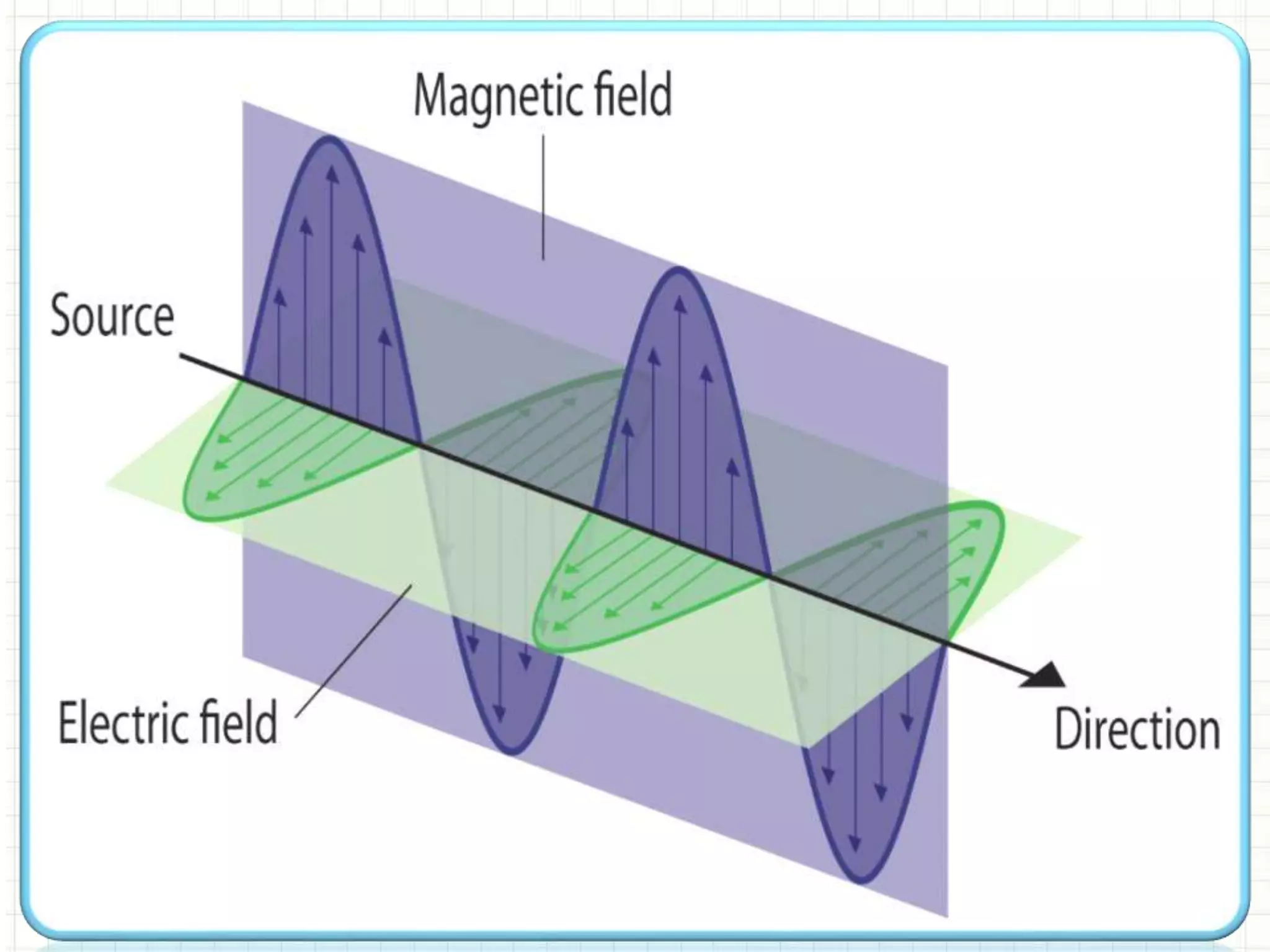
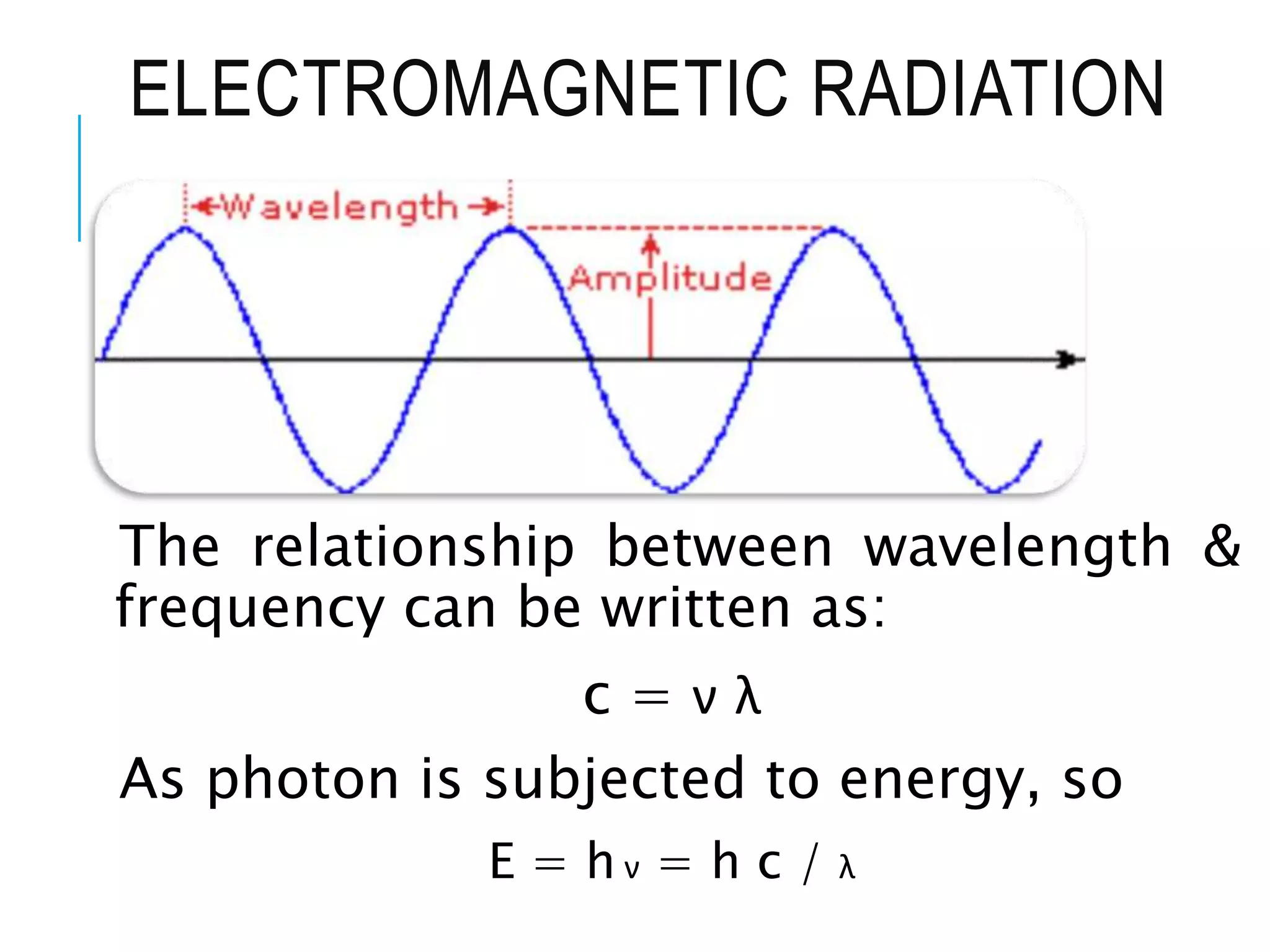
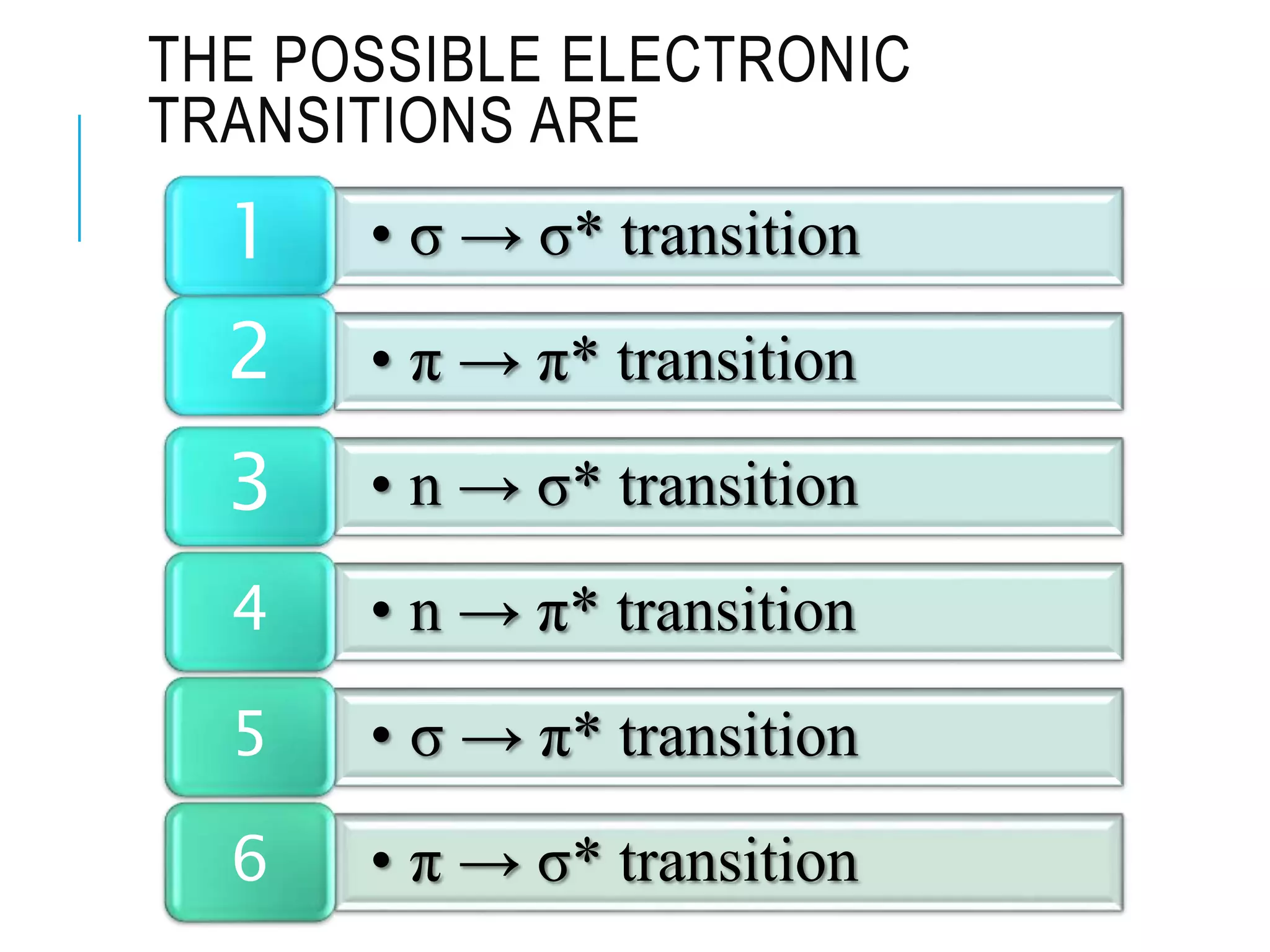
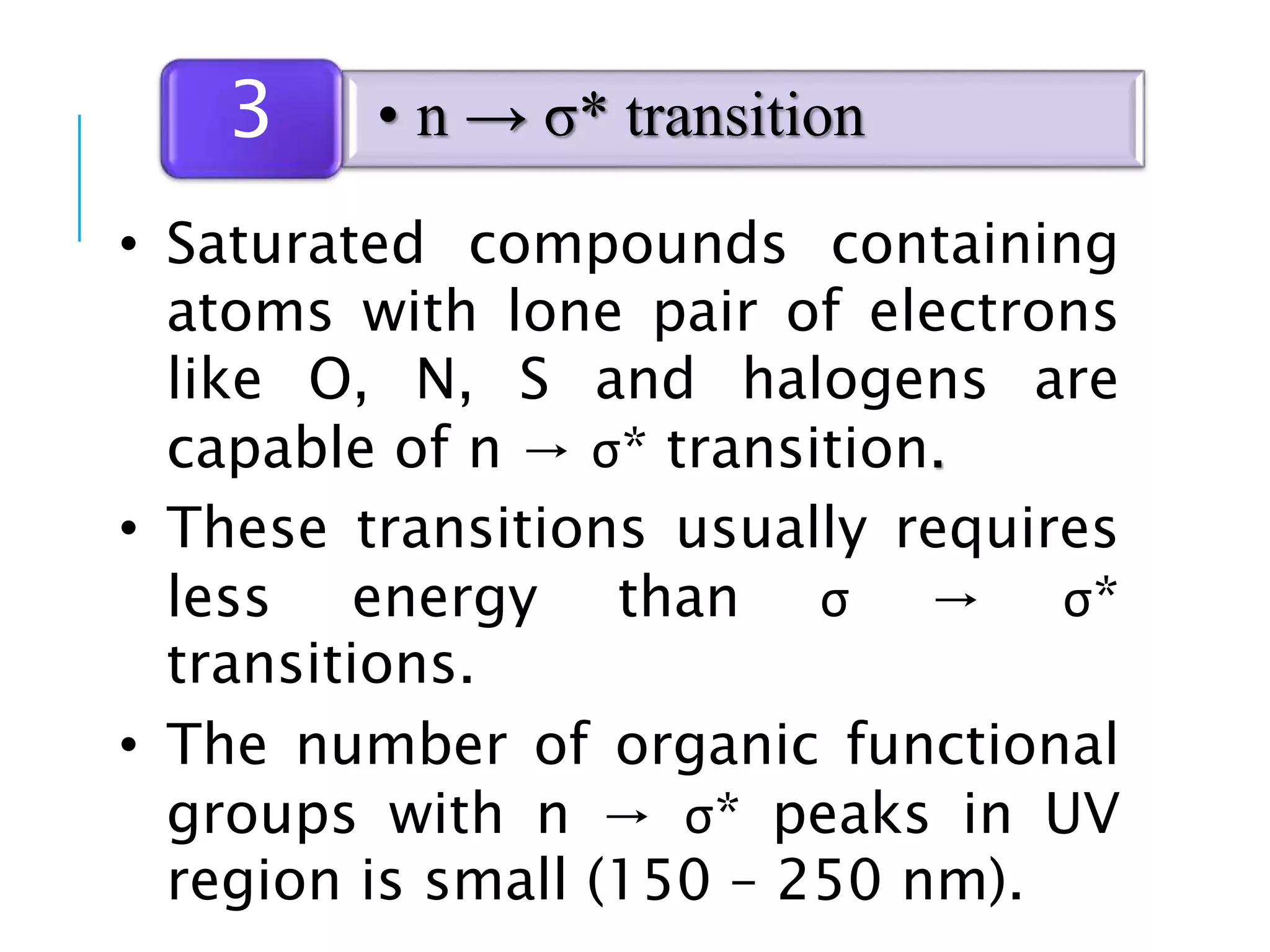
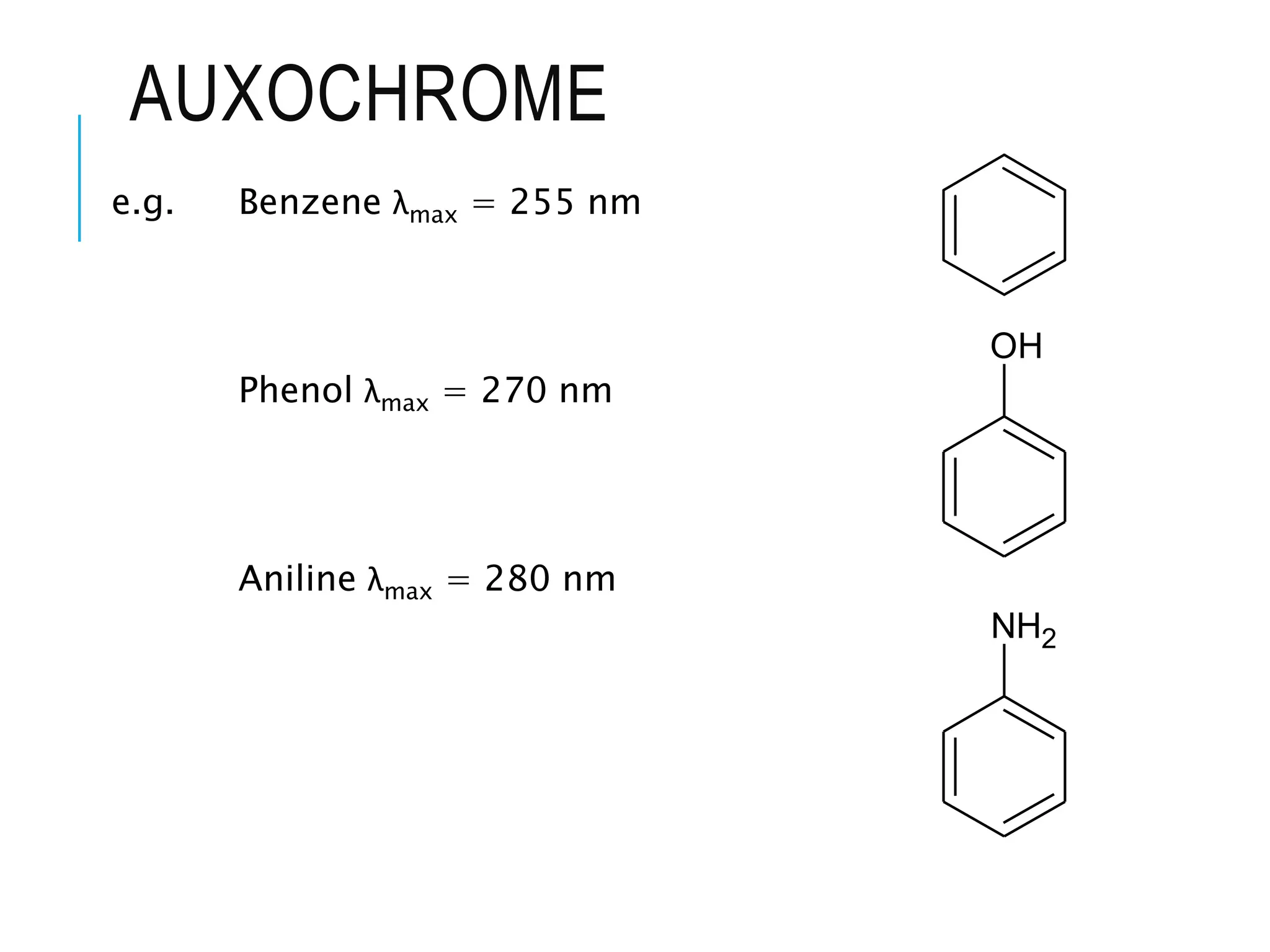
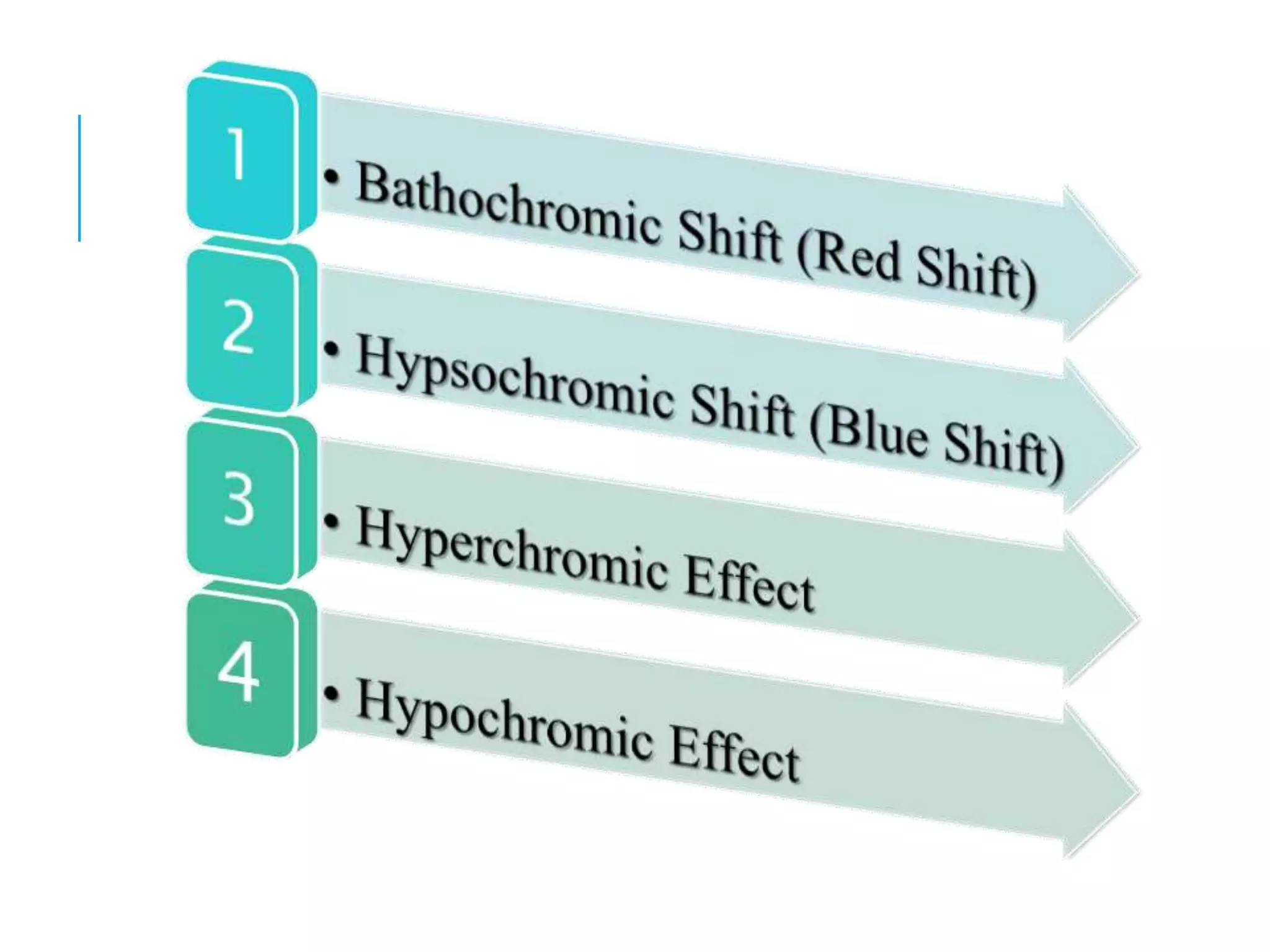
This document provides information about spectroscopy. It defines spectroscopy as the study of interaction of electromagnetic radiation with matter. It discusses the different types of electromagnetic radiation including ultraviolet-visible spectroscopy, infrared spectroscopy, and mass spectroscopy. It focuses on ultraviolet-visible spectroscopy, explaining that it involves electronic transitions when molecules absorb ultraviolet or visible light. It describes factors that affect absorption spectra such as chromophores, auxochromes, and solvents. It also defines terms used in ultraviolet-visible spectroscopy and discusses the types of shifts and effects that can occur in absorption spectra.