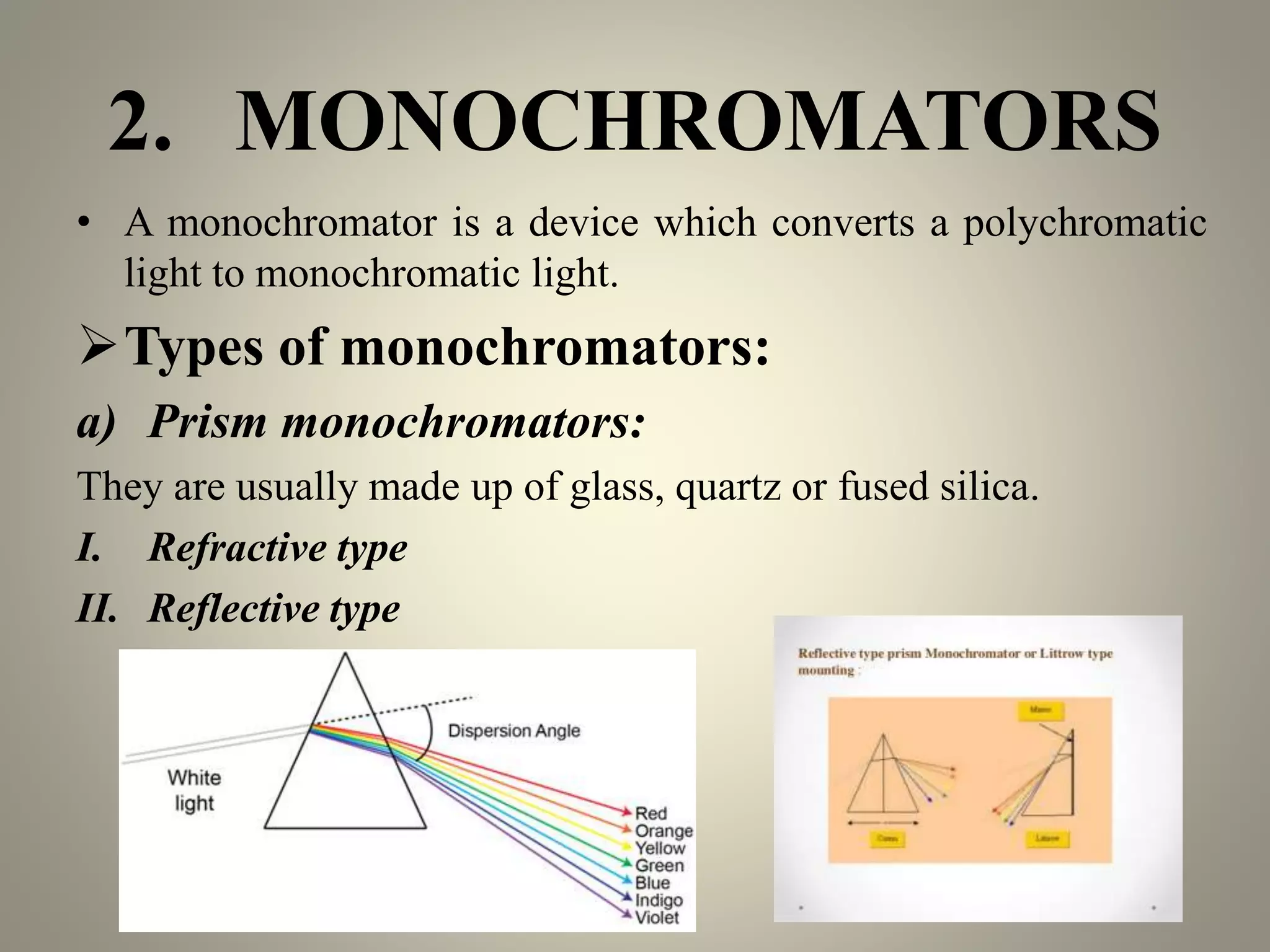
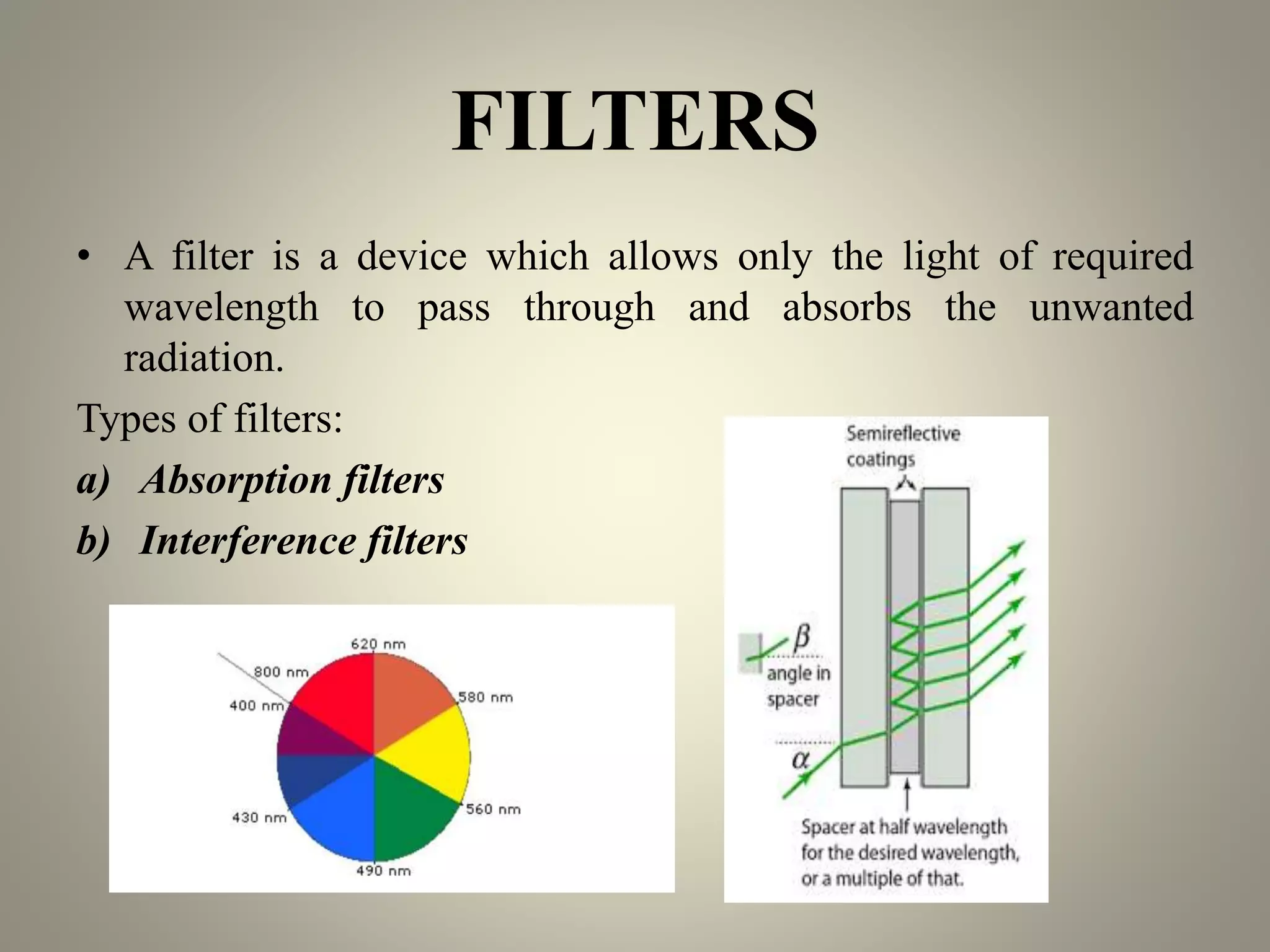
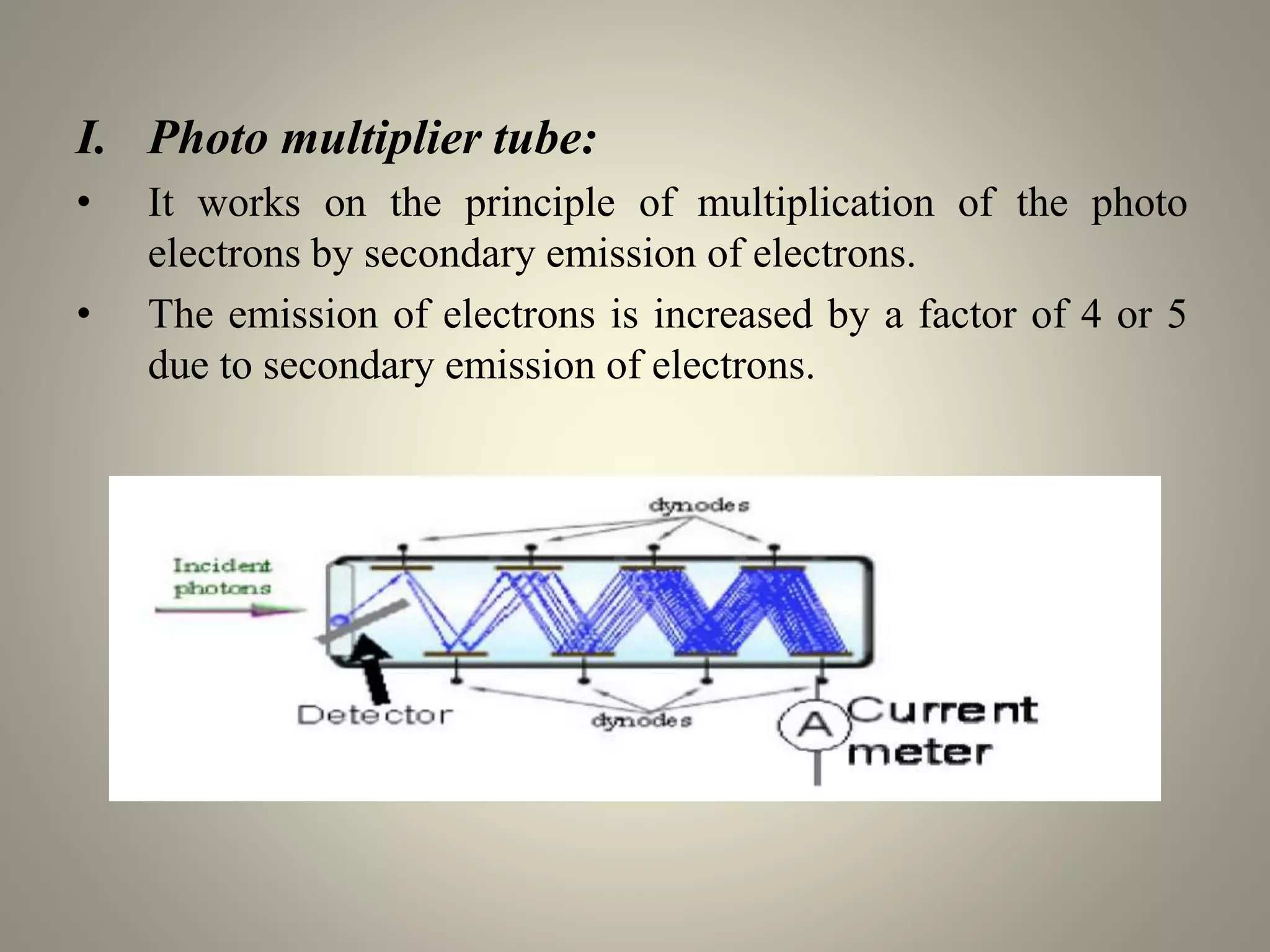
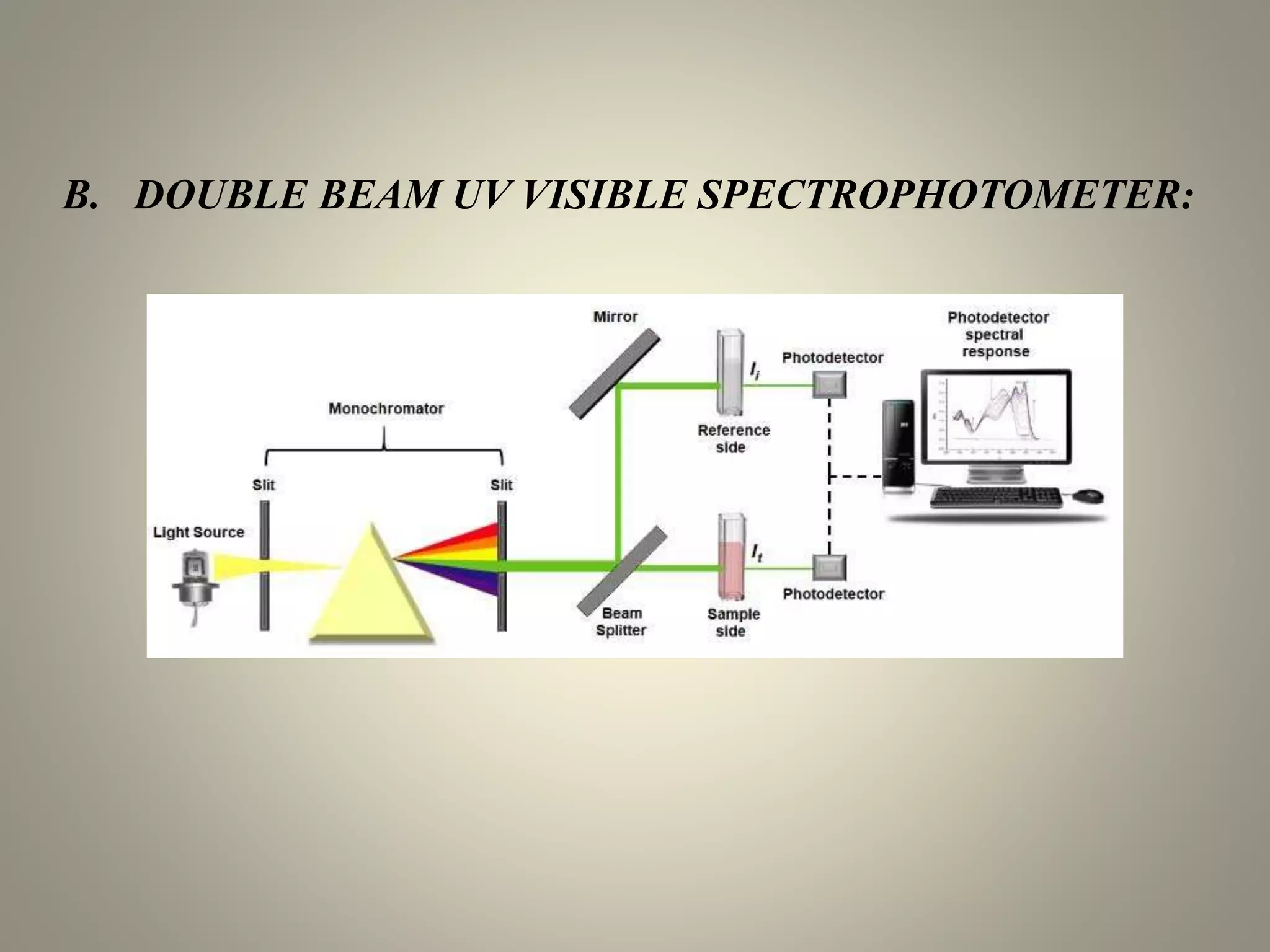
UV-visible spectroscopy is a technique that uses light in the visible and adjacent ranges. It works by measuring how much light is absorbed by a sample at each wavelength. There are several types of electronic transitions that can occur when molecules absorb this light. The amount of light absorbed follows Beer's law and is proportional to the concentration and path length of the sample. A UV-visible spectrophotometer consists of a light source, monochromator, sample holder, detector, and recording device. This technique has many applications including detection of impurities, structure elucidation, and quantitative analysis in pharmaceutical analysis.