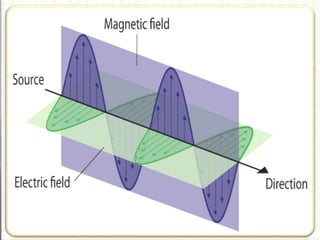
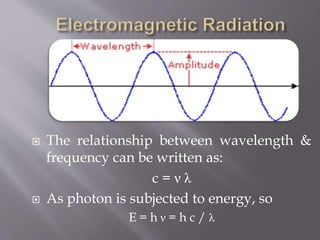
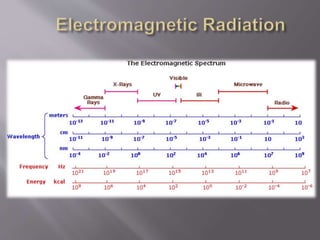
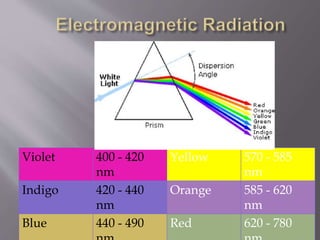
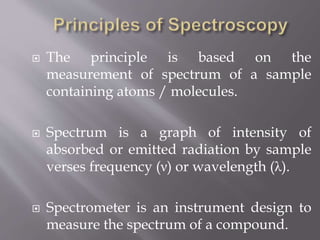
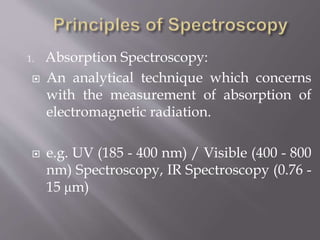
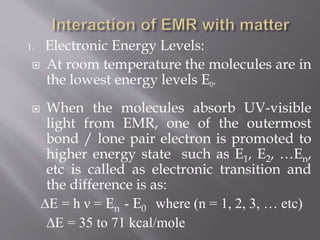
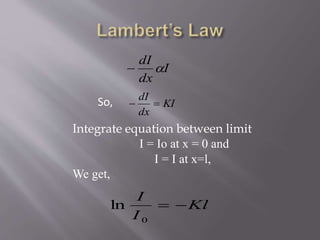
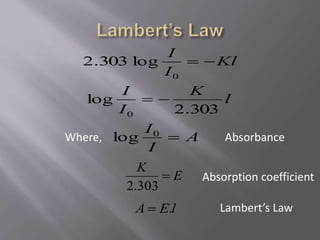
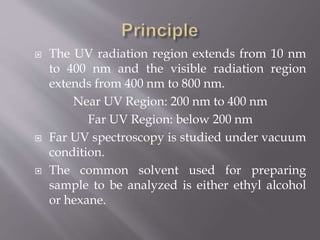
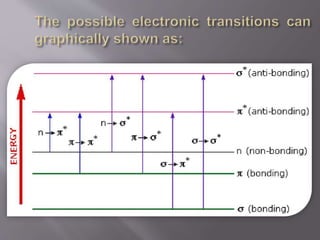
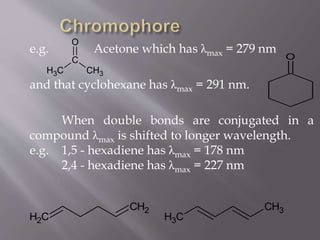
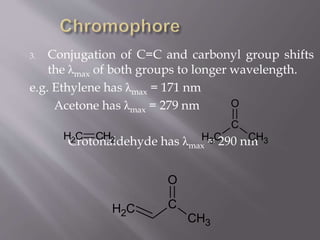
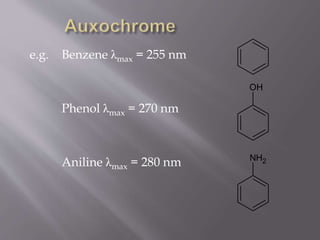
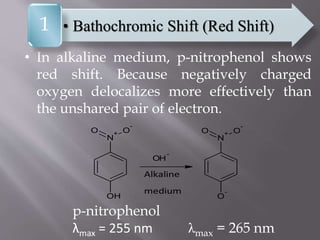
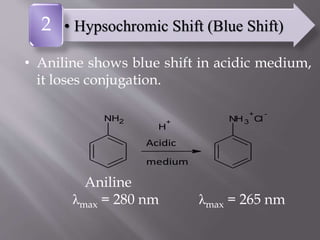
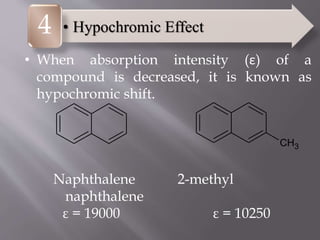
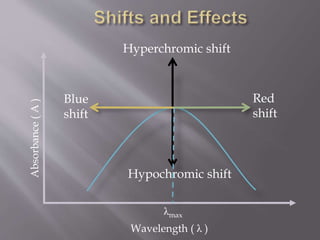
UV spectroscopy involves the interaction of ultraviolet or visible light with matter. It works on the principle that when UV or visible light hits a molecule, electrons within the molecule can be excited to higher energy levels. This causes absorption of specific wavelengths of light that are characteristic of a particular chemical bond or structure. The wavelength of absorbed light and intensity of absorption can be used to identify molecules and determine concentration.