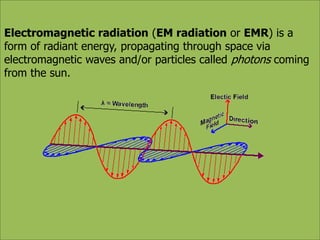
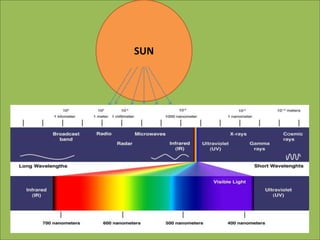
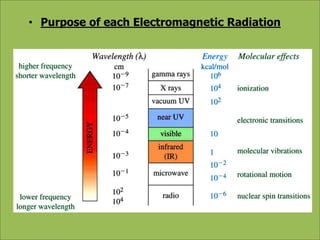
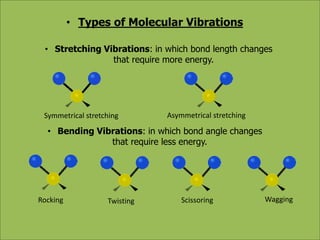
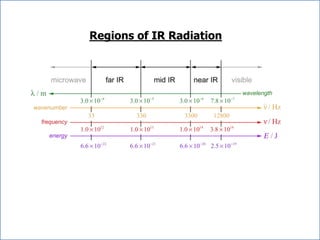
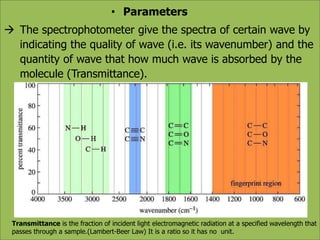
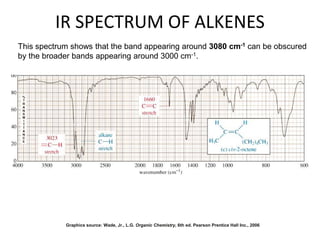
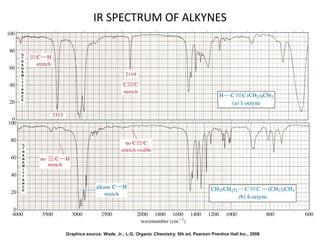
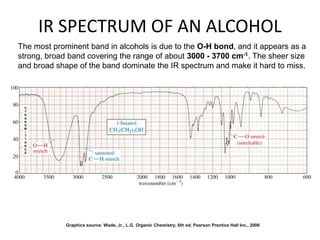
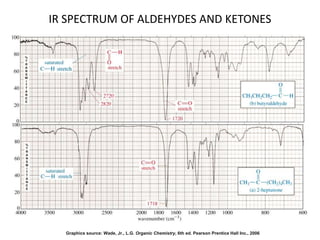
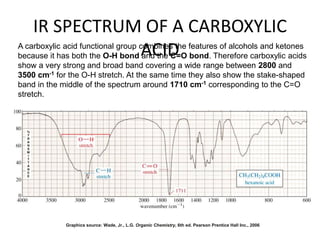
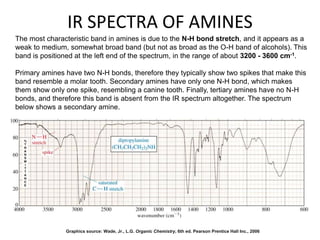
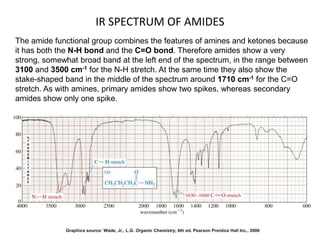
This document discusses using infrared spectroscopy to determine the structure of organic compounds. It begins by explaining electromagnetic radiation and the infrared region. It describes the different types of molecular vibrations that can be observed in an infrared spectrum. The document then explains how to interpret an infrared spectrum, noting the functional group and fingerprint regions. It provides examples of interpreting spectra for specific functional groups such as alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic acids, amines and amides. Key absorption bands that identify each functional group are highlighted.