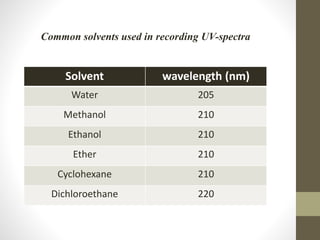
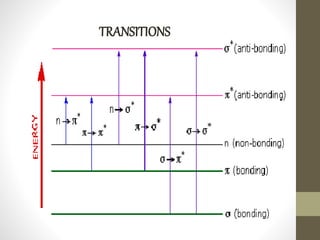
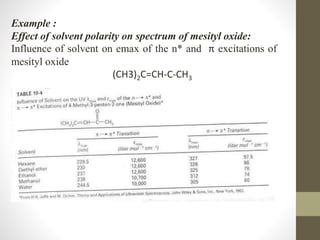
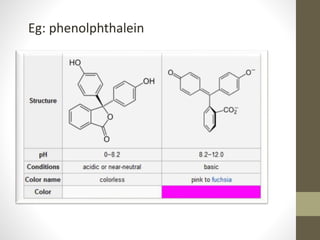
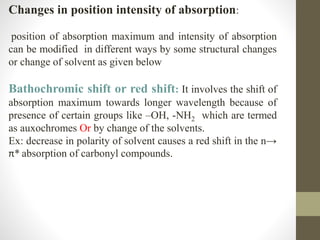
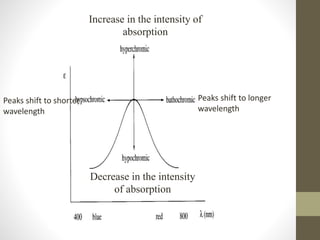
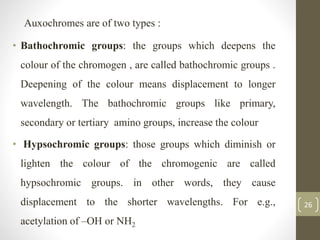
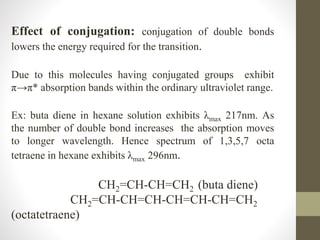
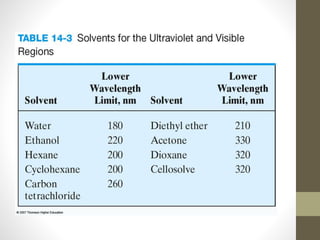
The document discusses how solvents and chromophores affect UV-visible spectroscopy. It states that the solvent exerts influence on the absorption spectrum, with the same drug showing different absorption maxima in different solvents. Common solvents used are water, methanol, ethanol, ether, and cyclohexane. The solvent should not absorb in the region studied and have minimum interaction with solute. Chromophores like conjugated systems, carbonyls, and metal complexes determine absorption. Factors like conjugation, auxochromes, and solvent polarity can shift absorption maxima.