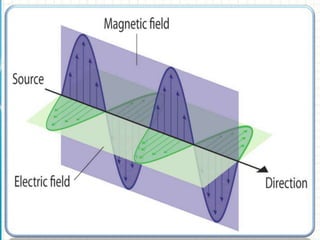
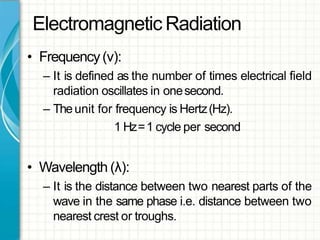
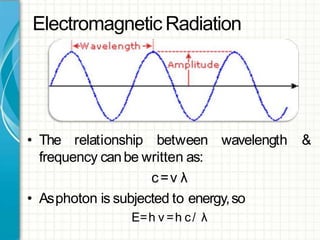
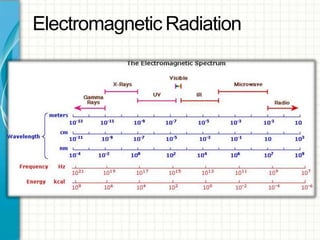
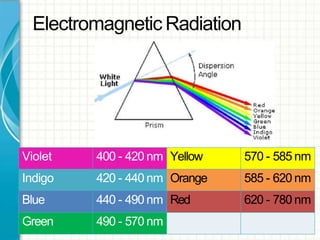
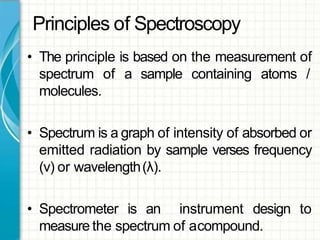
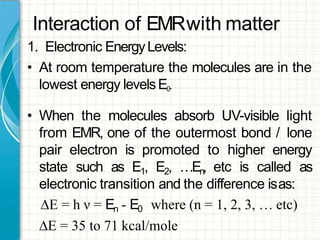
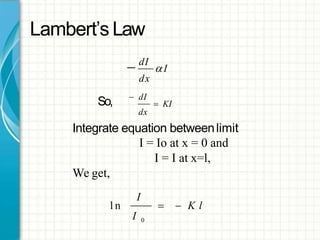
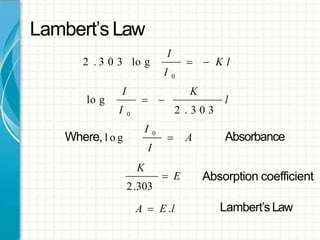
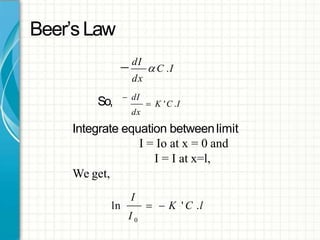
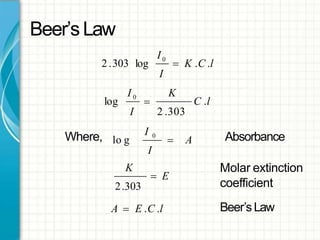
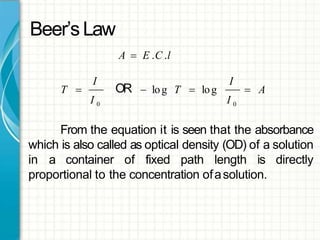
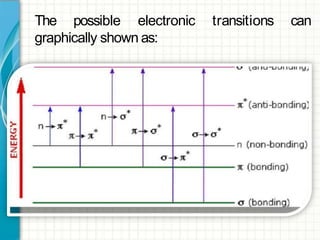
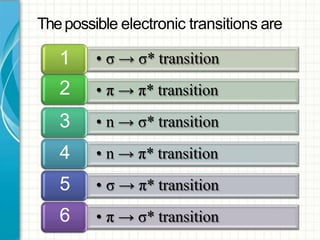
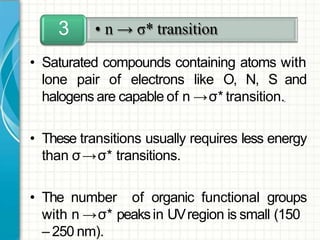
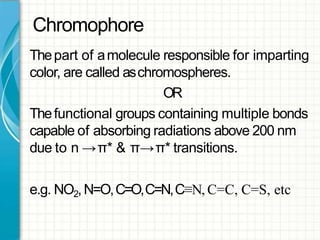
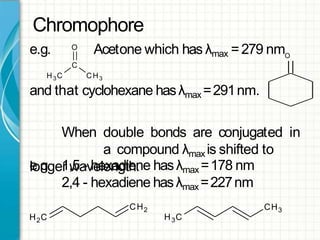
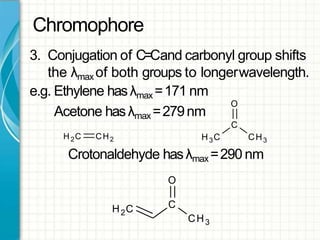
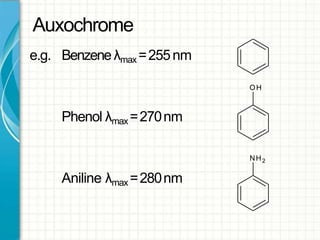
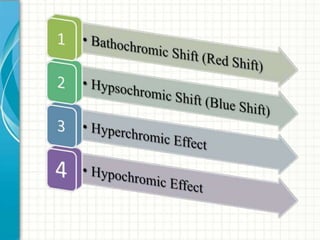
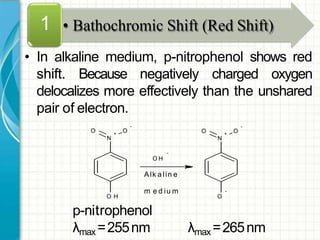
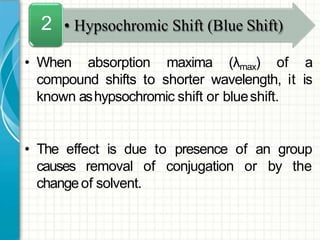
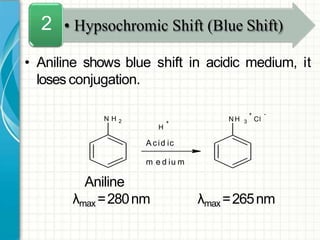
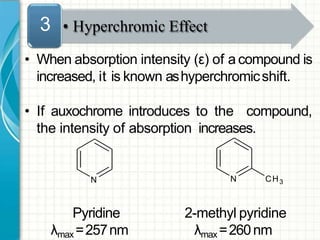
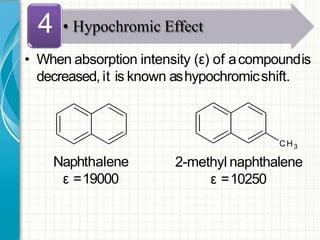
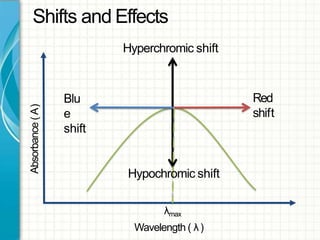
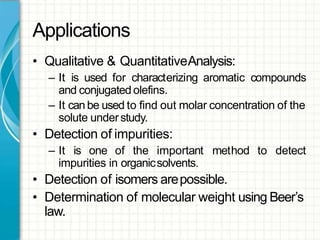
UV/visible spectroscopy involves using electromagnetic radiation in the UV and visible light range to analyze samples. Key principles are that different functional groups and molecular structures absorb radiation at characteristic wavelengths. Absorption of light causes electronic transitions between molecular orbitals. The Beer-Lambert law states that absorbance is directly proportional to concentration, with molar absorptivity coefficients describing this relationship. Absorption spectra provide information to identify compounds and determine concentrations.