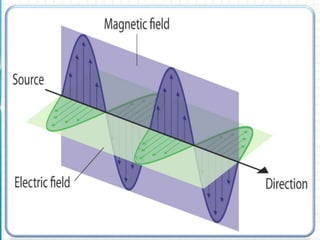
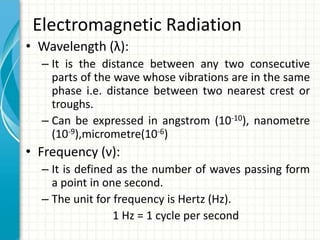
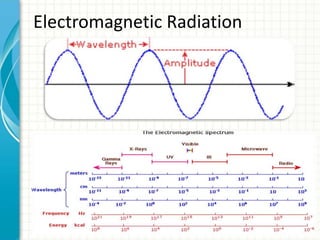
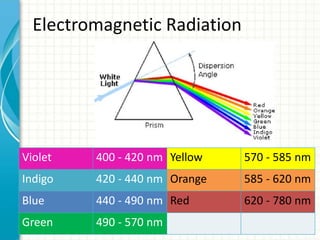
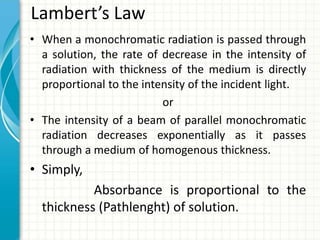
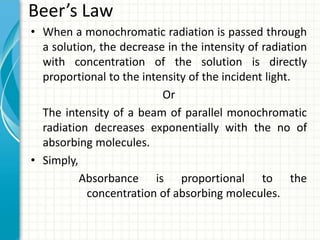
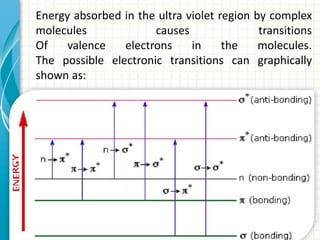
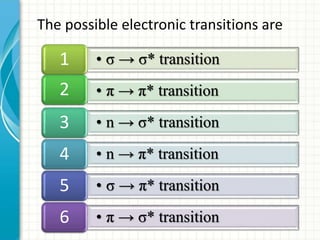
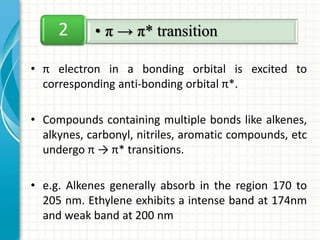
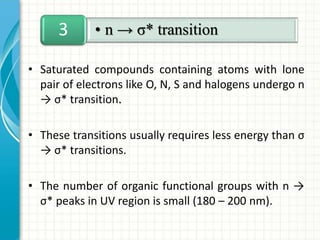
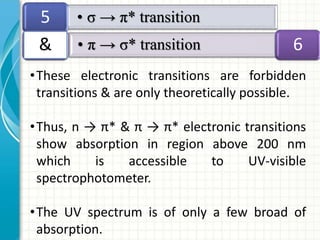
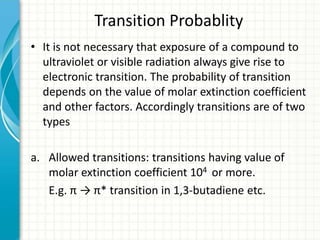
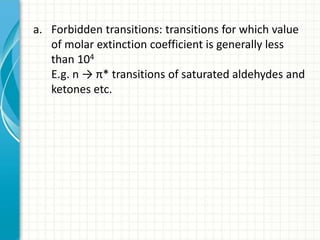
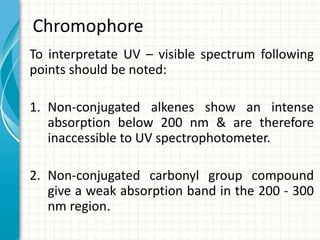
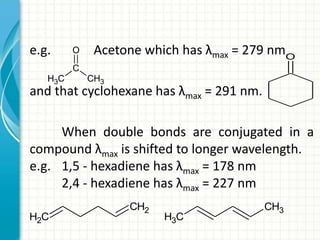
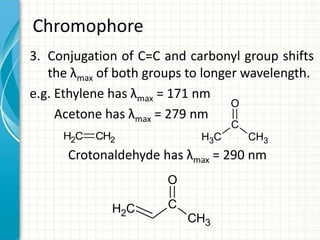
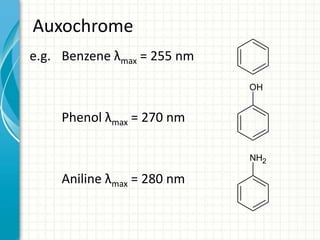
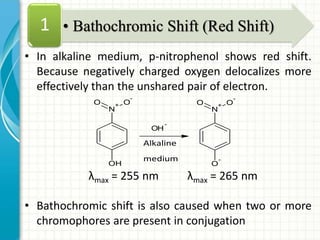
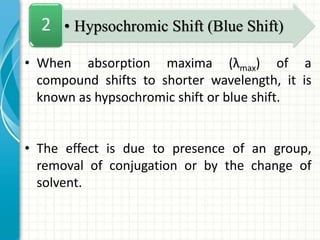
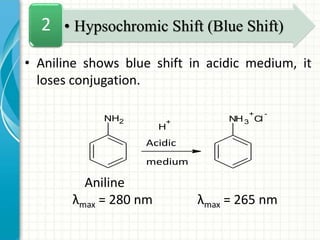
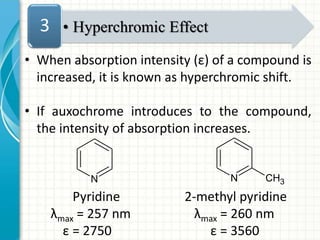
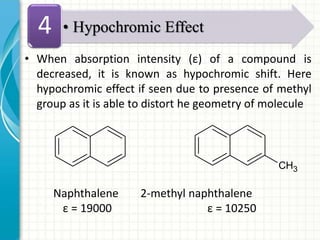
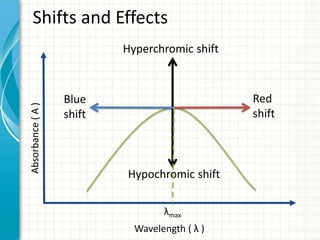
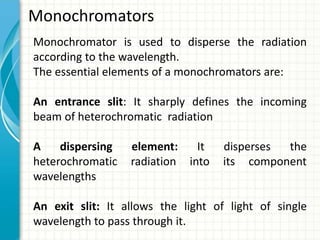
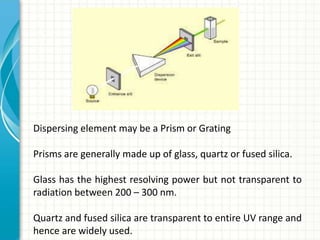
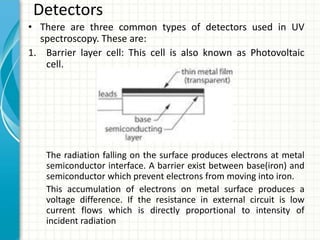
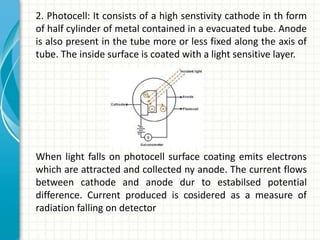
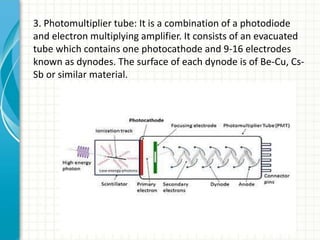
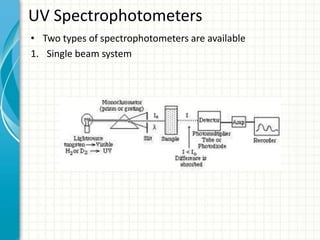
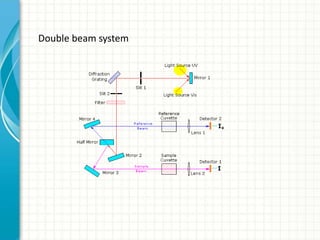
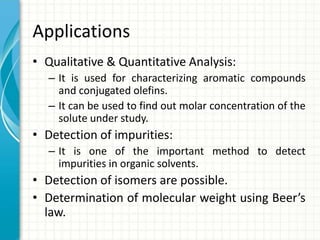
Spectroscopy is the study of interaction of electromagnetic radiation with matter. It involves measuring the spectrum (absorption or emission) of a sample when it interacts with electromagnetic radiation such as visible light, UV light, or infrared light. The main types of spectroscopy are absorption spectroscopy and emission spectroscopy. UV-visible spectroscopy measures absorption of ultraviolet and visible light by a substance in solution. It follows Beer-Lambert law where absorbance is directly proportional to concentration and path length of light through the sample. Electronic transitions that occur when absorbing UV-visible light include σ→σ*, n→π*, π→π*, etc. Factors like auxochromes, conjugation, and solvents can cause shifts in the absorption maximum