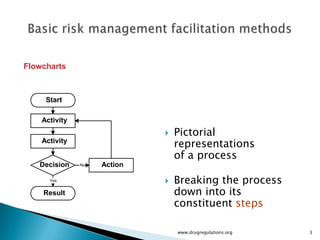
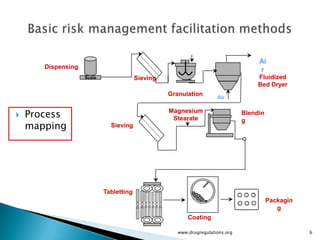
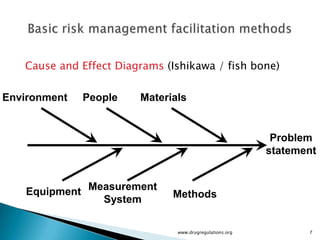
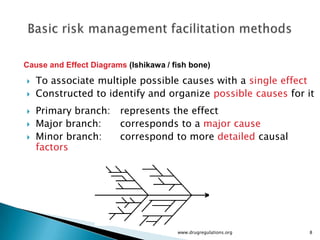
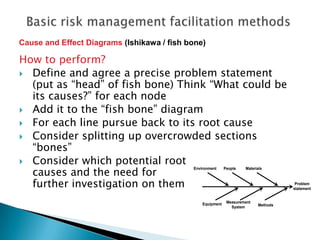
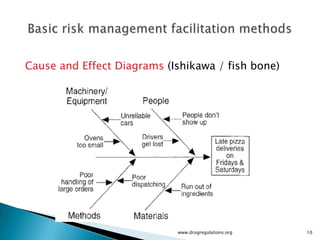
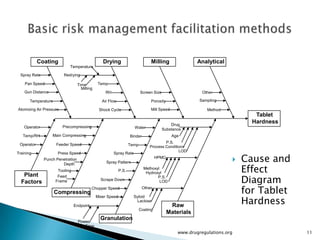
The document outlines fundamental methods for quality risk management in pharmaceuticals, including tools such as flowcharts, check sheets, process mapping, and cause-and-effect diagrams. These techniques facilitate risk identification and understanding of complex processes related to drug manufacturing. Additionally, it emphasizes the importance of accurately defining problem statements to identify root causes effectively.