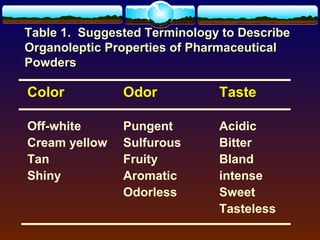
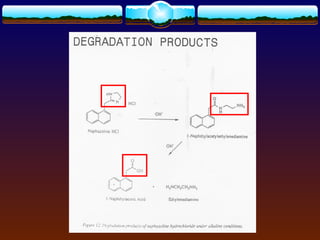
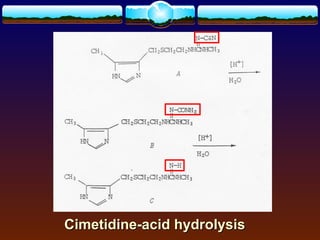
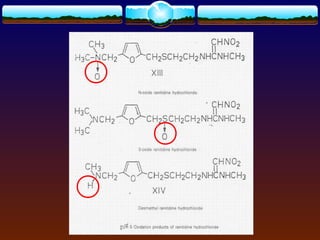
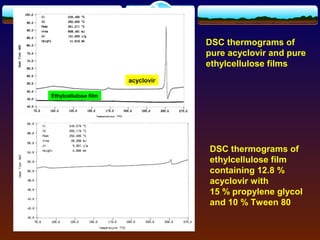
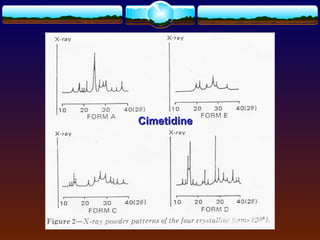
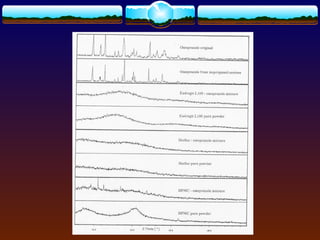
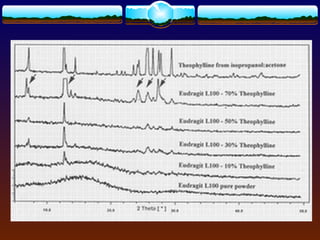
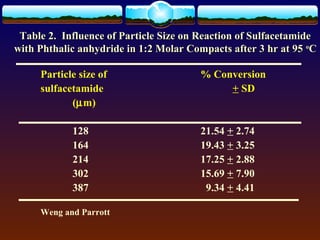
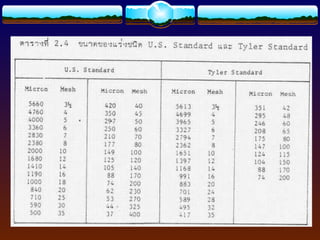
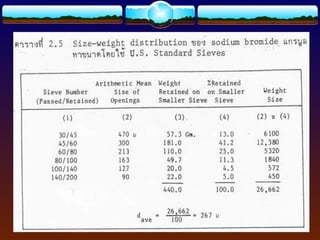
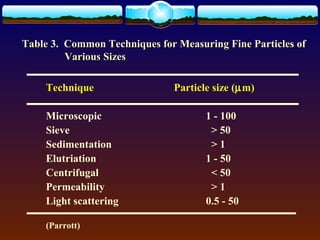
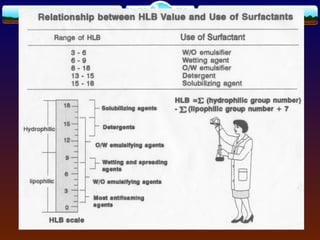
The document discusses preformulation testing, which is the first step in developing solid dosage forms and involves investigating a drug's physical and chemical properties alone and with excipients to generate useful information for formulating stable and bioavailable dosage forms. It outlines various characterization tests and properties that should be evaluated including solubility, particle size, purity, and surface area to guide formulation development and ensure batch-to-batch consistency.



















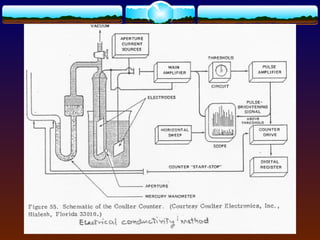
![3.1.3 Electronic means
To encompass most pharmaceutical
powders ranging in size 1 - 120 µm:
- Blockage of electrical conductivity path
(Coulter)
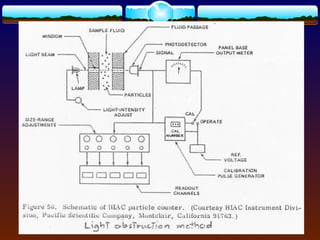
- Light blockage (HIAC) [adopted by USP]
- Light scattering (Royco)
- Laser scattering (Malvern)](https://image.slidesharecdn.com/preformulationtestingofsoliddosageforms-121130114303-phpapp01/85/Preformulation-testing-of-solid-dosage-forms-35-320.jpg)



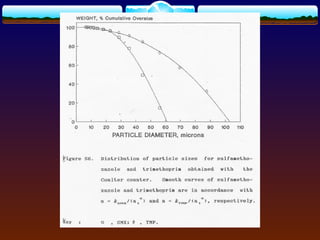



![ The values of λ (g of adsorbate/g of adsorbent) at
various P values (partial pressure of the adsorbate gas)
could be obtained from the experiment through
instrument.
Po (vapor pressure of the pure adsorbate gas) can be
obtained from the literature.
Plotting the term 1/[λ(Po/P - 1)] against P/Po will obtain
a straight line with
slope = (C - 1)/λmC
intercept = 1/λmC
The term C and λm can readily be obtained](https://image.slidesharecdn.com/preformulationtestingofsoliddosageforms-121130114303-phpapp01/85/Preformulation-testing-of-solid-dosage-forms-52-320.jpg)





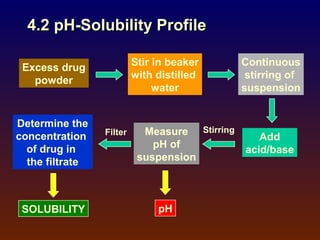
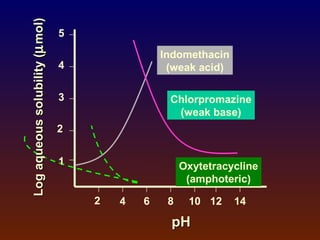
![ Poorly-soluble weakly-acidic drugs:
pH = pKa + log [(St - So)/So] (2)
Poorly-soluble weakly-basic drugs:
pH = pKa + log [So/(St - So)] (3)
where
So = solubility of unionized free acid or base
St = total solubility (unionized + ionized)](https://image.slidesharecdn.com/preformulationtestingofsoliddosageforms-121130114303-phpapp01/85/Preformulation-testing-of-solid-dosage-forms-63-320.jpg)


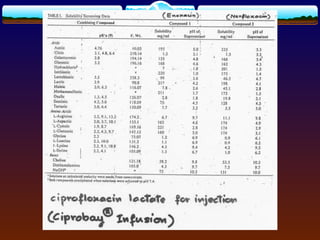

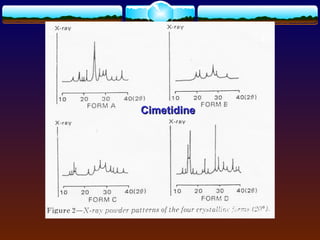
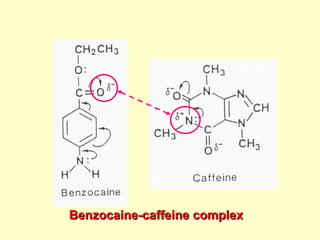
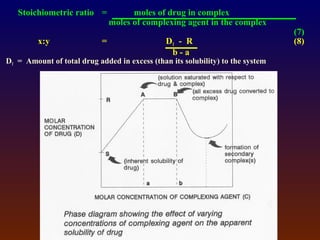
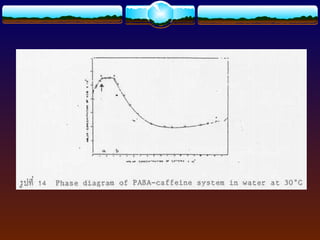
![4.4.1 Complexation
Complexation can be analyzed and explained on
the basis of “law of mass action” as follows:
D (solid) D (solution) (4)
xD + yC DxCy (5)
St = [D] + x[DxCy] (6)
where
D = drug molecule
C = complexing agent (ligand)
St = total solubility of free drug [D] and the
drug in the complex [DxCy]](https://image.slidesharecdn.com/preformulationtestingofsoliddosageforms-121130114303-phpapp01/85/Preformulation-testing-of-solid-dosage-forms-70-320.jpg)











![Henderson-Hasselbalch equation
For acids:
pH = pKa + log [ionized form]/[unionized form]
For bases:
pH = pKa + log [unionized form]/[ionized form]
Determination of Ionization Constant
1. Potentiometric pH-Titration
2. pH-Spectrophotometry Method
3. pH-Solubility Analysis](https://image.slidesharecdn.com/preformulationtestingofsoliddosageforms-121130114303-phpapp01/85/Preformulation-testing-of-solid-dosage-forms-100-320.jpg)