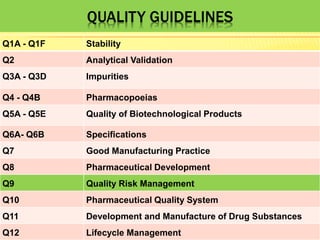
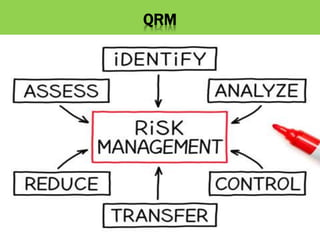
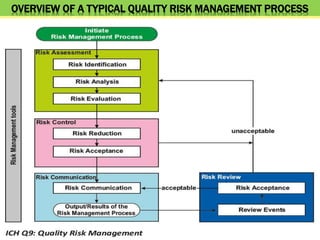
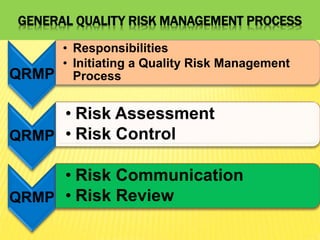
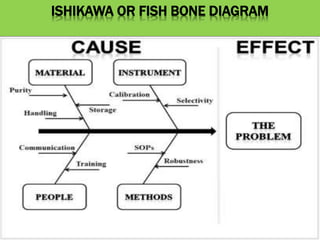
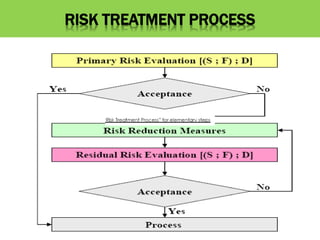
This document discusses quality risk management as outlined in the ICH Q9 guideline. It provides an introduction to quality risk management, including definitions of risk and management. It then describes the general quality risk management process, which involves responsibilities, initiating a risk assessment, risk assessment, risk control, risk communication, and risk review. Finally, it discusses various risk management tools and methods and provides potential applications of quality risk management in different aspects of the pharmaceutical quality system.