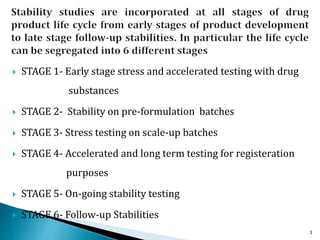
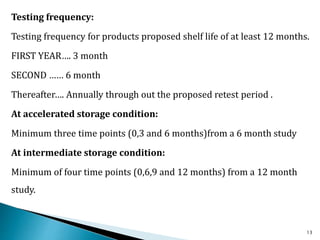
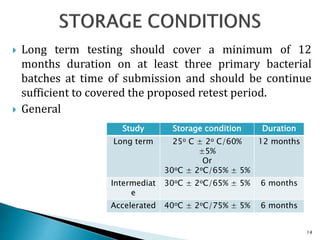
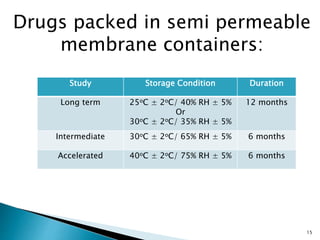
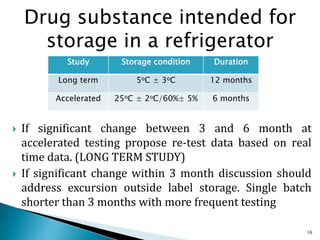
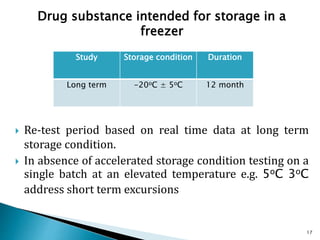
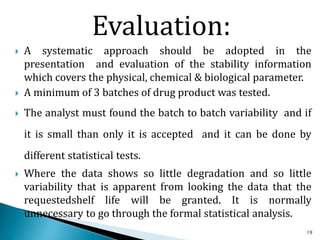
1. Drug stability testing involves conducting studies under various temperature, humidity and light conditions to determine a drug's shelf life and optimal storage requirements.
2. The ICH Q1A guideline provides the standard process for stability testing new drug substances and products to obtain registration. It defines testing stages, storage conditions and frequencies to evaluate how quality varies over time.
3. Stability testing helps establish expiration dates and provides evidence for appropriate packaging and labeling to ensure drug quality through a product's shelf life.