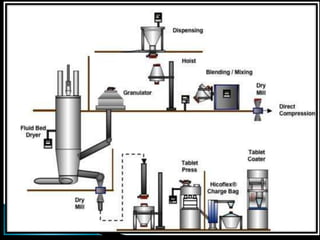
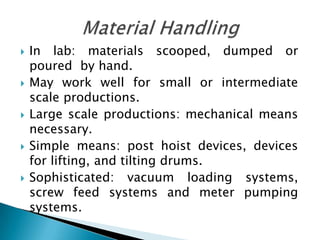
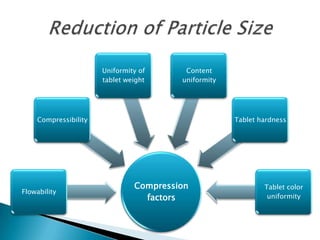
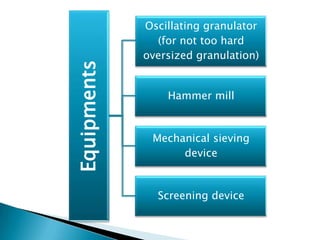
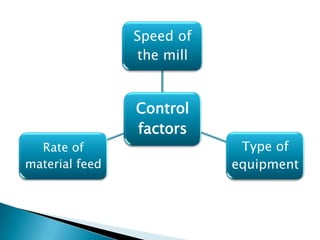
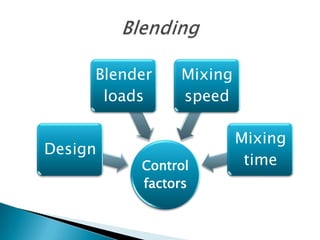
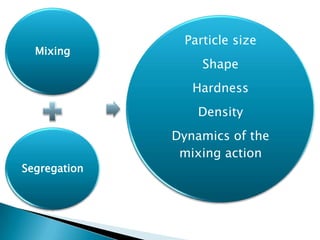
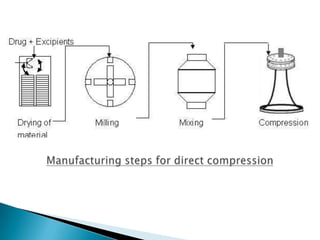
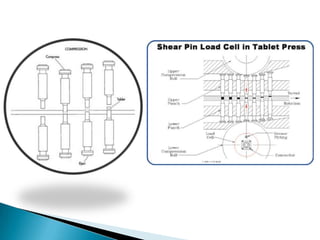
This document discusses the design and scale up considerations of pilot plants for tablet manufacturing. It begins by defining a pilot plant and its significance in transforming a lab-scale formula into a viable product. The objectives of the pilot plant include producing stable dosage forms, identifying critical process features, and providing information to support larger scale production. The document then discusses various unit operations involved in tablet manufacturing like material handling, blending, granulation, drying, milling, blending, compression, and coating. It provides details on equipment selection and process parameters that must be considered during scale up for each unit operation to ensure quality and reproducibility at production scale.