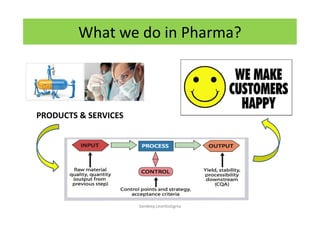
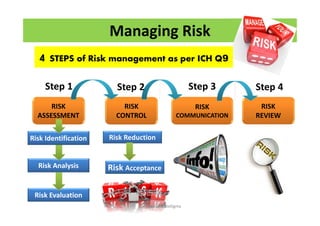
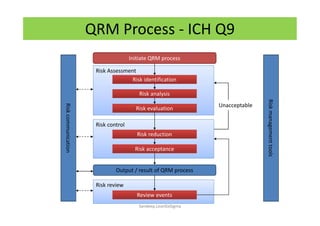
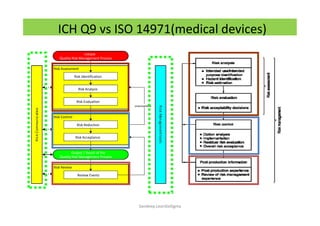
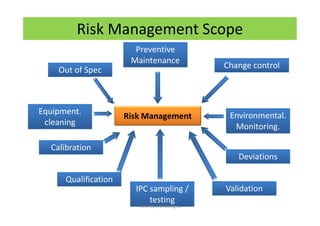
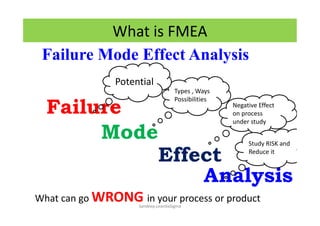
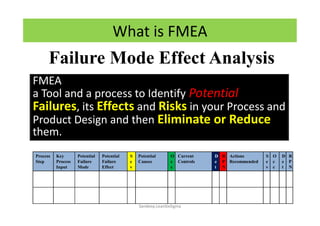
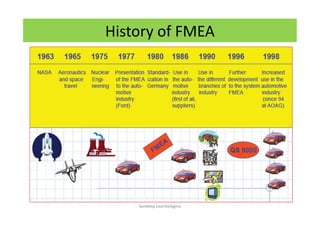
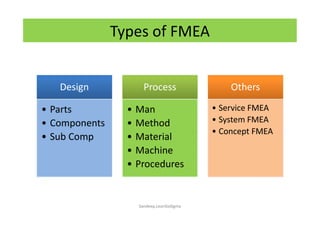
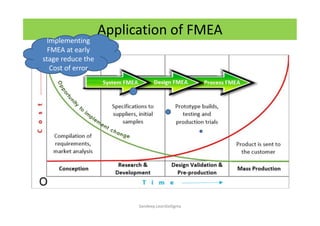
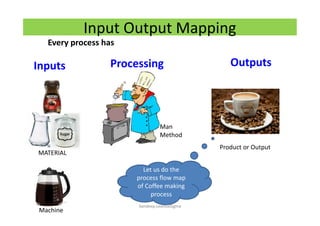
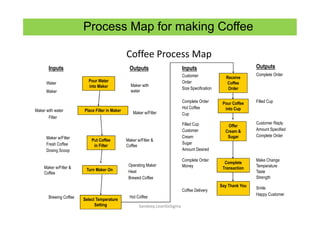
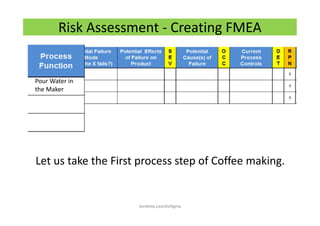
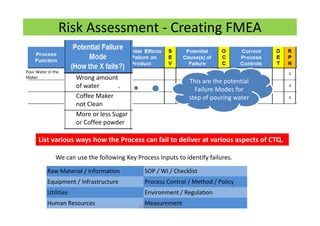
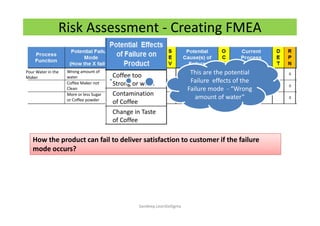
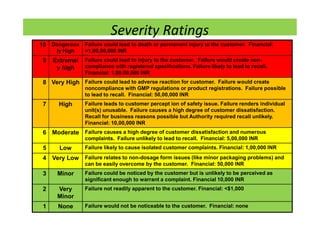
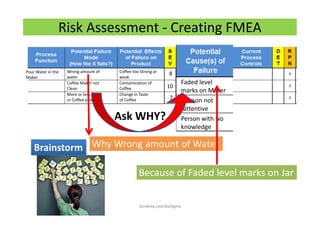
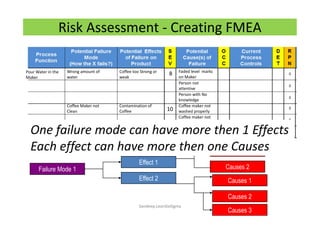
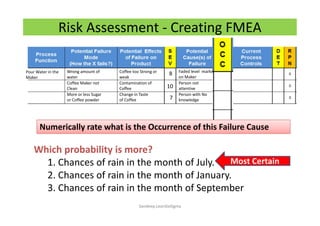
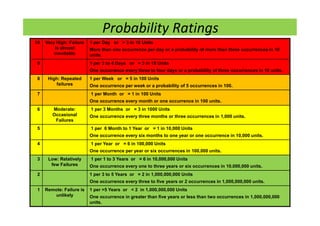
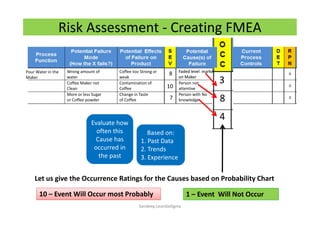
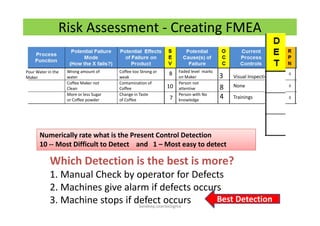
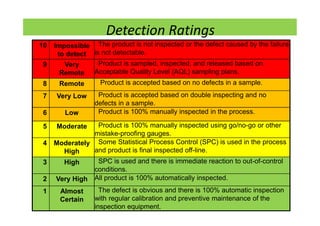
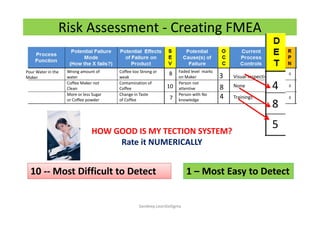
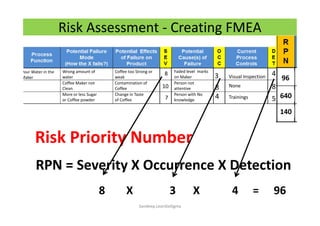
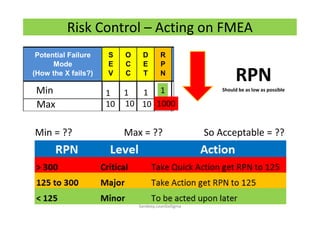
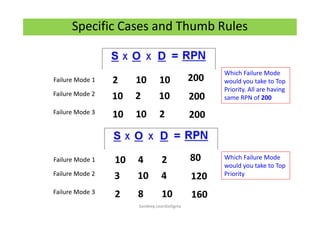
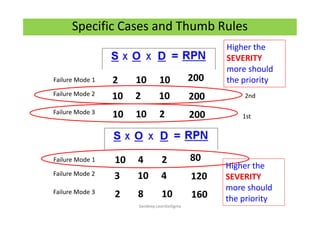
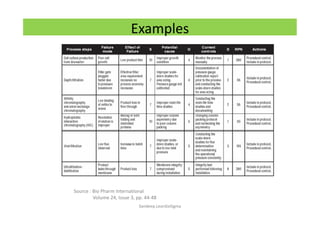
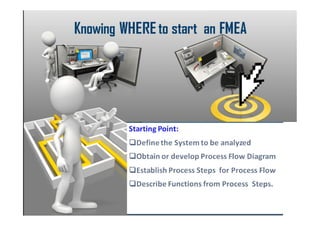
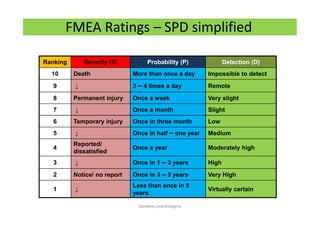
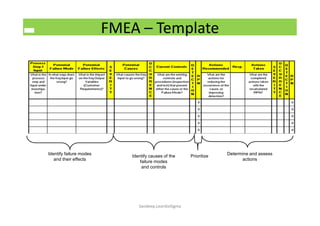
The document discusses risk management in the pharmaceutical industry using Failure Mode and Effects Analysis (FMEA) as a key tool. It outlines a four-step risk management process according to ICH Q9, focusing on identifying potential failures and their impacts while providing practical examples like the coffee-making process. FMEA is presented as a systematic approach for evaluating risks, prioritizing them, and implementing controls to enhance product quality and compliance.