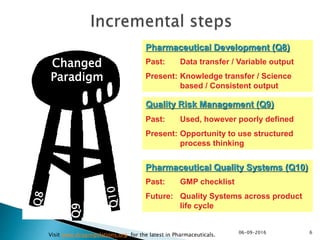
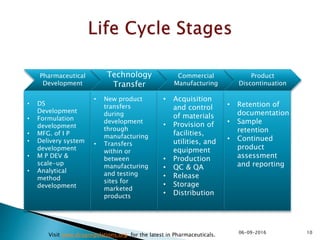
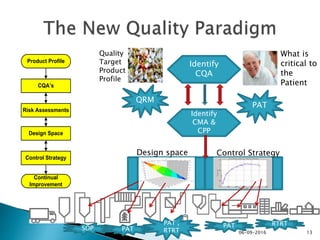
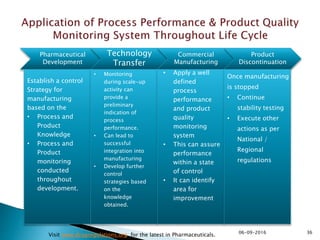
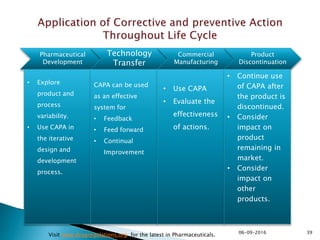
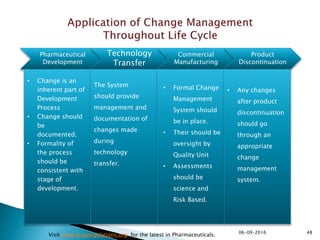
The document outlines the principles and guidelines for an effective pharmaceutical quality system based on ICH Q10, emphasizing the integration of quality risk management throughout the product lifecycle. It highlights the importance of management commitment, communication, and continual improvement in achieving quality objectives within the pharmaceutical sector. The content also describes the necessary systems for monitoring process performance and product quality, as well as the management of changes and outsourced activities.